Alcohols , Phenols and Ethers
Topics:Classification and Nomenclature of Alcohols, Phenols and Ethers
Classification of Alcohols and Phenols
- On the basis of number of hydroxyl groups
- Monohydric
- Dihydric
- Polyhydric (tri-, tetra-, etc.)
- Monohydric alcohols are classified on the basis of hybridisation of C in C−OH bond.
- Compounds containing
−OH bond
Further classified as −
- 1°, 2° and 3° alcohols
- Allylic alcohols
- Benzylic alcohols
- Compounds containing
−OH bond
Further classified as −
- Vinylic alcohol
CH2=CH−OH
- Phenols
Classification of Ethers
Two categories −
- Simple or symmetrical
- The two alkyl or aryl groups attached to the oxygen atom are the same.
- Mixed or unsymmetrical
- The two alkyl or aryl groups attached to the oxygen atom are different.
Nomenclature
- Alcohols
- The common names are derived from the common name of the alkyl group, with the word alcohol added to it.
- The IUPAC names are derived by substituting ‘e’ of the alkane (from which the alcohol is derived) with the suffix ‘−ol’.
- Common and IUPAC names of some alcohols are listed in the given table.
Compound
|
CH3OH
| ||
Common name
|
Methyl alcohol
|
Isopropyl alcohol
|
tert-Butyl alcohol
|
IUPAC name
|
Methanol
|
Propan-2-ol
|
2-Methylpropan-2-ol
|
- For naming cyclic alcohols, prefix ‘cyclo’ is used
- Phenols
Common and IUPAC names of some phenols are given below.
Compound
|
Common name
|
IUPAC name
|
Phenol
|
Phenol
| |
o-Cresol
|
2-Methylphenol
| |
Catechol
|
Benzene-1,2-diol
| |
Resorcinol
|
Benzene-1,3-diol
| |
Hydroquinone or Quinol
|
Benzene-1,4-diol
|
- Ethers
- The common names are derived from the alkyl or aryl groups by writing them as separate words and adding the word ‘ether’ at the end.
- Common and IUPAC names are listed in the given table.
Compound
|
Common Name
|
IUPAC name
|
CH3OCH3
|
Dimethyl ether
|
Methoxymethane
|
CH3OCH2CH2CH3
|
Methyl n-propyl ether
|
1-Methoxypropane
|
C6H5OCH2CH3
|
Ethylphenyl ether
|
Ethoxybenzene
|
C6H5OCH3
|
Methylphenyl ether (Anisole)
|
Methoxybenzene (Anisole)
|
C6H5O(CH2)6 − CH3
|
Heptylphenyl ether
|
1-Phenoxyheptane
|
Example :
Calculate the ratio of percentage of p − character in the carbon atom which is attached directly to − OH in allylic, vinylic and benzylic alcohol respectively?
- A )
2 : 2 : 3
- B )
3 : 3 : 2
- C )
3 : 2 : 3
- D )
2 : 3 : 3
(i) Allylic alcohol: 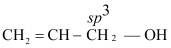
(ii) Vinylic alcohol: 
(iii) Benzylic alcohol:
The ratio of percentage p − character in (i), (ii) and (iii) is
Example :
Which is the correct structure of 4 − methoxyacetophenone?
- A )
- B )
- C )
- D )
4 − Methoxyacetophenone contains two functional groups - ether and ketone.
−OCH3 is methoxy which is an ether.
−COCH3 is aceto which is a ketone.
In IUPAC nomenclature, ketone is given preference over ether.
Topics:Methods of Preparation of Alcohols and Phenols
Preparation of Alcohols
- From alkenes
- By acid-catalysed hydration
Mechanism:
Step 1 − Protonation of alkene by electrophilic attack of H3O+ to form carbocation
Step 2 − Nucleophilic attack of water on the carbocation
Step 3 − Deprotonation to form alcohol
- By hydroboration−oxidation
The product so formed looks as if it were formed by the addition of water to the alkene in a way opposite to Markovnikov’s rule.
- From carbonyl compounds
- By reduction of aldehydes and ketones
Catalysts → finely divided metals such as Pt, Pd or Ni, NaBH4, LiAlH4
Aldehydes give 1° alcohol while ketones give 2° alcohol
- By reduction of carboxylic acids and esters
Since LiAlH4 is an expensive reagent, alcohol is produced from carboxylic acid commercially in another manner.
- From Grignard reagents
- Methanal gives 1° alcohol
- Other aldehydes give 2° alcohol
- Ketones give 3° alcohol
Preparation of Phenols (Also Known as Carbolic Acid)
- From haloarenes
- From benzenesulphonic acid
- From diazonium salts
- From cumene
Example :
Which of the following compounds on reacting with Grignard reagents yields a primary alcohol?
- A )
CH3COCH3
- B )
CH3CHO
- C )
HCHO
- D )
All of the above
Primary alcohol is formed when Grignard reagents react with formaldehyde (HCHO). This reaction occurs in two steps.
Step-I: Nucleophilic addition of Grignard reagents to carbonyl group (> C = O) to form an adduct
Step-II: Hydrolysis of the adduct to yield a primary alcohol
The mechanism of the reaction is shown as follows.
All other aldehydes give secondary alcohols whereas ketones give tertiary alcohol with Grignard reagents.
Example :
Consider the following reaction:
The products P and Q respectively are
- A )
- B )
- C )
- D )
Topics:Physical Properties of Alcohols and Phenols
Boiling Points
- Increase with the increase in number of carbon atoms
- Reason − With the increase in the number of carbon atoms, van der Waals forces increase.
- Decrease with increase of branching
- Reason − With the increase in branching, surface area decreases and hence, van der Waals forces decrease.
- Alcohols and phenols have higher boiling points than other classes of compounds (hydrocarbons, ethers, haloalkanes, and haloarenes) of comparable molecular masses.
- Reason − They undergo extensive intermolecular hydrogen bonding resulting in aggregation of molecules.
Solubility
- Soluble in Water
- Reason − They undergo H-bonding with water molecules.
Example :
Consider the given compounds.
(p)
(q)
(r)
(s)
The correct order of boiling points of the given compounds is
- A )
q < r < s < p
- B )
r <p < q < s
- C )
r < q < p < s
- D )
q < s < r < p
It is observed that the boiling points of alcohols and phenols are higher than aldehydes, ethers and hydrocarbons of comparable molecular masses. This is because of intermolecular hydrogen bonding which is absent in hydrocarbons and ethers.
Example :
Consider the following compounds.
(I) Phenol
(II) 2 − methylphenol
(III) 3 − methylphenol
(IV) 4 − methylphenol
Which is the correct order of boiling points of the given compounds?
- A )
(I) < (III) < (II) < (IV)
- B )
(I) < (II) < (III) < (IV)
- C )
(IV) < (III) < (II) < (I)
- D )
(IV) < (II) < (III) < (I)
The boiling point of phenols increases with increase in molecular mass of phenol. Among o−, m− and p− isomers, as the distance between −OH and CH3− increases, intermolecular hydrogen bonding becomes easy and hence, boiling point increases.
Topics:Chemical Reactions of Alcohols and Phenols
- Alcohols act both as nucleophiles and electrophiles.
- Alcohols as nucleophiles
- Protonated alcohols as electrophiles
Reactions Involving Cleavage of O−H Bond
- Acidity of alcohols and phenols
- Reaction with active metals such as Na, K and Al
- Phenols react with aq. NaOH to form sodium phenoxides.
- Acidity of phenols
- Acidic character arises due to the polar nature of O−H
- The acid strength of alcohols increases in the order
- Alcohols are weaker acids than water.
- Alcohols act as Bronsted bases as well.
- Phenols are stronger acids than alcohols.
Reason:
In alcohol, −OH is attached to the sp3 hybridised carbon whereas in phenol, −OH is attached to the sp2 hybridised carbon. Since sp2 hybridised carbon is more electronegative thansp3hybridised carbon, electron density on the oxygen atom in phenol decreases. As a result, the polarity of O−H bond increases, and hence, ionisation of phenol increases. This leads to increase in the acidity of phenols.
Phenoxide ion is more stable than alkoxide ion due its resonance stabilisation.
Therefore, phenol is more acidic than alcohol.
- Electron-withdrawing substituents (especially at ortho- and para- positions) increase the acidity of phenols whereas electron-donating substituents decrease acidity.
- Esterification
- Example − Acetylation of salicylic acid
Reactions Involving Cleavage of C−O Bond in Alcohols
- Reaction with hydrogen halides:
- Lucas test [test with Lucas regent (conc. HCl and ZnCl2)] − Used for distinguishing the three classes of alcohols.
- Reaction with phosphorus trihalides:
- Dehydration:
From 1° alcohol
From 2° alcohol
From 3° alcohol
Therefore, the order reactivity can be observed as
1° < 2° < 3°
- Mechanism of dehydration
Step 1 − Formation of protonated alcohols
Step 2 − Formation of carbocation
Step 3 − Formation of alkene by elimination of a proton
- Oxidation: Involves formation of a carbon−oxygen double bond, with cleavage of O−H and C−H bonds
- Also known as dehydrogenation as dihydrogen is lost.
- Depending on the oxidising agent, a primary alcohol is oxidised to aldehydes and then to carboxylic acid.
Oxidising agents such as potassium dichromate converts primary alcohol to aldehydes and resist further oxidation.
- Strong oxidising agents such as acidified KMnO4 convert alcohols directly into carboxylic acid.
- Reagents such as CrO3 in anhydrous medium, PCC (pyridinium chlorochromate) are used for isolation of aldehydes.
- 2° alcohols are converted into ketones by CrO3
- 3° alcohols do not undergo oxidation; however, under strong oxidising agents such as KMnO4 and high temperature, a mixture of carboxylic acids containing lesser number of carbon atoms is formed.
- Reaction with heated copper at 573 K
Example :
Use the following information to answer the next question.
(i)
(ii)
|
In the above reactions alcohol is behaving as
- A )
nucleophile in (i) and electrophile in (ii)
- B )
electrophile in (i) and nucleophile in (ii)
- C )
nucleophile in both (i) and (ii)
- D )
electrophile in both (i) and (ii)
Alcohol has the ability to behave both as nucleophile and electrophile.
When alcohols behave as nucleophile, the bond between O − H is broken. This is depicted by reaction (ii).
On the other hand, when alcohols behave as electrophile, the bond between C − O is broken. This is depicted by reaction (i).
Example :
Consider the following reaction.
In the given reaction, X, Y and Z respectively are
- A )
X → 2 − Butanol; Y →2−methyl − 2 − butanol; Z − 2 − Butanone
- B )
X → 2 − methyl − 2 − butanol; Y → 2 − Butanol; Z → 2 − Butanone
- C )
X → 2 − methyl − 2 − butanol; Y → − 2 − Butanone; Z → 2 − Butanol
- D )
X → 2 − Butanol; Y → 2 − Butanone; Z→ 2 − methyl − 2 − butanol
C4H10O (X) may be ether or alcohol. Since it gives alkene at 443 K, it is an alcohol. Since (Y) gives positive iodoform test, it must be methyl ketone (CH3COCH2CH3).
This is possible only when (X) is 2 − Butanol.
Ketone with Grignard reagents only gives tertiary alcohol (Z). Thus, the following reactions take place.
Topics:Reactions of Phenols
Electrophilic Aromatic Substitution
- The −OH group activates the benzene ring towards electrophilic substitution and directs the incoming group to ortho- and para- positions.
- Nitration
- With dilute HNO3
The o- and p-isomers can be separated by steam distillation.
Reason: p-nitrophenol is less volatile due to the association of molecules by intermolecular H-bonding, while o-nitrophenol is steam volatile due to intramolecular H-bonding.
- With concentrated HNO3:
- Halogenation
- Reaction carried out in solvents such as CS2 or CHCl3:
- With bromine water:
Kolbe’s Reaction
Reimer-Tiemann Reaction
Reaction with Zinc Dust
Oxidation
The presence of phenol can be confirmed by converting it to phenolphthalein which turns pink in basic solution.Example :
Which of the following compounds can undergo bromination even in the absence of iron (III) bromide?
- A )
- B )
- C )
- D )
All of the above
The presence of −OCH3, −NH2 and −OH groups on benzene ring cause ring activation. A Lewis acid is required to polarise the halogen molecule. However, in case of phenol, methoxybenzene and aniline, polarisation of halogen molecule (Br2) takes place even in the absence of Lewis acid.
Example :
In which reaction aspirin is obtained from acetic anhydride?
- A )
Reimer Tiemann reaction
- B )
Kolbe's reaction
- C )
Kolbe’s electrolysis reaction
- D )
Rosenmund reduction reaction
The product of Kolbe’s reaction is salicylic acid which on acetylation with (CH3CO)2 O (acetic anhydride) in concentrated H2SO4 results in formation of aspirin (acetylsalicylic acid).
Topics:Some Commercially Important Alcohols
Methanol (CH3OH)
- Known as wood spirit
- Preparation
- Earlier produced by destructive distillation of wood
- Catalytic hydrogenation of carbon monoxide
CO + 2H2 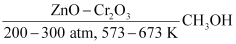
- Properties
- Colourless liquid
- Boiling point = 337 K
- Highly poisonous − Small quantities cause blindness and large quantities cause even death.
- Uses
- As a solvent in paints and varnishes
- In the preparation of formaldehyde (HCHO)
Ethanol (C2H5OH)
- Preparation
- By fermentation
C12H22O11 + H2O  C6H12O6 + C6H12O6
C6H12O6 + C6H12O6
Molasses Glucose Fructose
C6H12O6  2C2H5OH + 2CO2
2C2H5OH + 2CO2
Glucose/Fructose
When the percentage of alcohol formed exceeds 14%, the action of zymase is inhibited.
Fermentation takes place in absence of air. If air gets into fermentation mixture, then ethanol is oxidised to ethanoic acid by the oxygen present in air and as a result, the taste of alcoholic drink is destroyed.
- By hydration of ethane
- Properties
- Colourless liquid
- Boiling point = 351 K
- Uses
- As a solvent in manufacture of paint and a number of carbon compounds
- Denaturation of alcohol − Commercial alcohol becomes unfit for drinking by mixing some copper sulphate (to give it a colour) and pyridine (a foul smelling liquid). This is known asdenaturation of alcohol.
Example :
Wood spint is
- A )
HCHO
- B )
CH3OH
- C )
CH3CHO
- D )
CH3CH2OH
Methanol, CH3OH is known as wood spint. It is produced by catalytic hydrogenation of CO at high temperature and pressure.
Example :
Commercial alcohol is made unfit for drinking by mixing of
- A )
CuSO4 and 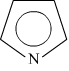
- B )
ZnSO4 and 
- C )
CuSO4 and 
- D )
ZnSO4 and 
Commercial alcohol is made unfit for drinking by adding some chemicals. This is known as denaturation of alcohol. Copper sulphate and pyridine is added in this method.
CuSO4 is added to provide the alcohol a colour while pyridine is added to give foul smelling.
Topics:Ethers
Preparation
- By dehydration of alcohols
- Alcohols undergo dehydration in the presence of protic acids like H2SO4, H3PO4.
- Product may be alkene or ether depending on the reaction conditions.
Example:
- Mechanism:
Formation of ether follows SN2 mechanism.
Step 1
Step 2
Step 3
- This method is applied to prepare ethers having primary alkyl groups only.
- When the alkyl group is 2° or 3°, elimination competes over substitution, and this leads to the formation of alkene.
- Williamson synthesis
- Better results are obtained if the alkyl halide is 1°. For 2° and 3° halides, elimination competes over substitution to form alkenes.
Reason:Alkoxides are nucleophiles as well as strong bases. They react with alkyl halides, leading to elimination reactions.
- This method can be used for converting phenols to ethers also.
Physical Properties
- Boiling point
Comparable to those of alkanes, but much lower than those of alcohols of comparable molecular mass
- Reason: Alcohols undergo intermolecular H−bonding while ethers do not
- Solubility
Soluble in water
- Reason: Form H−bonds with water
Chemical Reactions
- Cleavage of C−O bond in ethers
- React with excess of hydrogen halide under drastic conditions. Dialkyl ethers give two alkyl halide molecules.
- Alkyl aryl ethers react with hydrogen halide to give phenol and alkyl halide. Aryl−oxygen bond is not cleaved due to the high stability of aryl−oxygen bond.
- When the two alkyl groups are different, one alkyl halide molecule and one alcohol molecule is formed.
- The order of reactivity of hydrogen halide is
HCl < HBr < HI
- When one of the alkyl groups is tertiary, the alkyl halide is formed from the tertiary alkyl group.
- Electrophilic substitution
The alkoxy (−OR) group is ortho− and para− directing, and activates the aromatic ring towards electrophilic substitution due to resonance.
- Halogenation
- Friedel-Crafts alkylation
- Friedel-Crafts Acylation
- Nitration
Example :
Consider the following reaction.
Which of the following alternatives is correct about the products?
A )Bothalcohols and alkyl halides are primary.
B )Alcohol is tertiary whereas alkyl halide is primary.
C )Alcohol is primary whereas alkyl halide is tertiary.
D )Bothalcohols and alkyl halides are tertiary.
The mechanism of the reaction is
Step 1
Step 2
Step 3
In step 2, removal of CH3OH gives a tertiary carbocation [(CH3) 3C+ ] which is more stable. So the products of the reaction are primary alcohol and tertiary alkyl halide.
Example :
Consider the following reaction.
The product formed is
- A )
ether when R is primary
- B )
alkene when R is primary
- C )
alkene when R and R' are primary
- D )
ether when both R and R' are tertiary
The given reaction is Williamson synthesis of ethers.
The product is ether only when R is primary. In case of secondary and tertiary alkyl halides, alkene is the only product. This is because when R is primary, substitution reaction takes place but when R is secondary or tertiary, elimination reaction takes place. This difference in reaction mechanism arises of the fact that alkoxide (R' − O−) is not only a nucleophile but strong base too resulting in elimination reaction to give alkene.

BOC Sciences is constantly seeking to expand our product lines by incorporating additional potential drug like molecules. Moracin P
ReplyDeleteWhat are Alcohols Phenols and Ethers
ReplyDeleteThese three types of organic compounds are widely used in a variety of businesses and personal use. But what exactly are they?
When a saturated carbon atom links to a hydroxyl (-OH) group, the result is Alcohol.
When the -OH group replaces the hydrogen atom in benzene, we obtain Phenol.
When an oxygen atom links to two alkyl or aryl groups, Ether.
This blog post mainly focuses on the chapter; therefore, it is not required to read the whole chapter from the textbook. The following are the subtopics covered in Alcohols Phenols and Ethers for NEET notes: