Aldehydes, Ketones and Carboxylic Acids
Topics:Nomenclature and Structure of Carbonyl Group
Nomenclature
Common Names of Aldehydes
- Often called by their common names instead of IUPAC names
- Derived from the common names of the carboxylic acids by replacing the ending ‘−ic’ of the acid with aldehyde
- Location of the substituent in the carbon chain is indicated by the Greek letters α, β, γ, δ, etc.
- Example:
Common Names of Ketones
- Derived by naming two alkyl or aryl groups bonded to the carbonyl group
- Locations of substituents are indicated by the Greek Letters, α, α′, β, β′, and so on
- The simplest dimethyl ketone is called acetone.
- Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
IUPAC Names
- For open-chain aliphatic aldehydes and ketones, IUPAC names are derived from the names of the corresponding alkanes by replacing the ending ‘−e’ with ‘−al’ and ‘−one’ respectively.
- In the case of aldehydes, the longest chain is numbered starting from the carbon of the aldehydic group.
- In the case of ketones, the numbering begins from the end nearer to the carbonyl group.
- Substituents are prefixed in the alphabetical order along with the numerals indicating their positions in the carbon chain.
- Same rule is applicable to cyclic ketones.
- If the aldehydic group is attached to a ring, then the suffix carbaldehyde is added to the full name of cyclohexane.
Example:
- The common name benzaldehyde is also accepted by IUPAC.
- Common and IUPAC names of some aldehydes and ketones are listed in the given table.
Structure
|
Common name
|
IUPAC name
|
Aldehydes
| ||
HCHO
|
Formaldehyde
|
Methanal
|
CH3CHO
|
Acetaldehyde
|
Ethanal
|
(CH3)2CHCHO
|
Isobutyraldehyde
|
2-Methylpropanal
|
γ-Methylcyclohexane
|
3-Methylcyclohexanecarbaldehyde
| |
CH3CH(OCH3)CHO
|
α-Methoxypropionaldehyde
|
2-Methoxypropanal
|
CH3CH2CH2CH2CHO
|
Valeraldehyde
|
Pentanal
|
CH2= CHCHO
|
Acrolein
|
Prop-2-enal
|
Phthaldehyde
|
Benzene-1,2-dicarbaldehyde
| |
m-Bromophthaldehyde
|
3-Bromobenzene-1,2-dicarbaldehyde
| |
Ketones
| ||
CH3COCH2CH2CH3
|
Methyl n-propyl ketone
|
Pentan-2-one
|
(CH3)2CHCOCH(CH3)2
|
Diisopropyl ketone
|
2,4-Dimethylpentan-3-one
|
α-Methylcyclohexanone
|
2-Methylcyclohexanone
| |
(CH3)2C=CHCOCH3
|
Mesityl oxide
|
4-Methylpent-3-en-2-one
|
Structure of Carbonyl Group
- Carbonyl carbon atom is sp2 hybridised.
- It forms three sigma (σ) bonds and one pi (π) bond.
- The π bond is formed with oxygen by overlap with p-orbital of an oxygen atom.
- Oxygen atom has two non-bonding electron pairs.
- Carbonyl carbon and the three atoms attached to it lie in the same plane.
- π-electron cloud is above and below the plane.
- Bond angles are approximately 120° as is expected of a trigonal co-planar structure.
- Orbital diagram for the formation of carbonyl group is as follows:
- C=O double bond is polarised due to higher electronegativity of oxygen relative to carbon.
- Carbonyl carbon − an electrophile (Lewis acid)
- Carbonyl oxygen − a nucleophile (Lewis base)
- High polarity of the carbonyl group is explained on the basis of resonance involving neutral (A) and dipolar (B) structures as shown below.
Example :
The correct structure for 3-Propylcyclohexanecarbaldehyde is
- A )
- B )
- C )
- D )
The suffix ‘carbaldehyde’ is added to an aldehyde group attached to a ring. The numbering of carbon atoms constituting the ring start from the carbon atom attached to the aldehyde group.
Example :
Which of the following statements is not correct for carbonyl group > C = O?
- A )
C − O bond is polar in nature.
- B )
Bond angles are 120o with trigonal planar structure.
- C )
C is sp2 hybridized and forms 3σ bonds and 1π bond.
- D )
C acts as Lewis basic centre and O acts as Lewis acidic centre.
Carbon − oxygen bond is polar due to higher electronegativity of oxygen. So, C gets partial positive charge and O gets partial negative charge i.e. C becomes electron deficient or Lewis acid and acts as an electrophilic centre while O becomes electron rich or Lewis base and acts as a nucleophilic centre.
Example :
Which of the following pairs of compounds reacts together to form ?
?
- A )
- B )
and CrO2Cl2 in CS2
- C )
- D )
The reaction shown above is Etard reaction in which chromyl chloride (CrO2Cl2) in CS2 oxidises − CH3 to intermediate chromium complex. The structure of the complex is as follows.
On hydrolysis, the above compound gives benzaldehyde.
Topics:Preparation of Aldehydes and Ketones
Important Methods for the Preparation of Aldehydes and Ketones
- By oxidation of alcohols
Primary alcohols  Aldehydes
Aldehydes
Secondary alcohols  Ketones
Ketones
- By dehydrogenation of alcohols
Primary alcohols  Aldehydes
Aldehydes
Secondary alcohols  Ketones
Ketones
- From hydrocarbons
- Ozonolysis of alkenes followed by reaction with Zn dust and water gives aldehydes, ketones, or a mixture of both, depending upon the substitution pattern of the alkene.
- Hydration of alkynes
Other alkynes give ketones in this reaction.
Preparation of Aldehydes
- From acyl chloride (acid chloride)
- This reaction is called Rosenmund reduction.
- Example:
- From Nitriles
This reaction is called Stephen reaction.
Example:
- From esters
- Example:
- From hydrocarbons
- By oxidation of methyl benzene and its derivative using chromyl chloride (CrO2Cl2)
This reaction is called Etard reaction.
- By oxidation of methyl benzene and its derivative using chromic oxide (CrO3) in acetic anhydride
- By side chain chlorination followed by hydrolysis
- By Gatterman-Koch reaction
Preparation of Ketones
- From acyl chlorides
Treatment of acyl chlorides with dialkylcadmium gives ketones.
- From nitriles
Treatment of nitrile with Grignard reagent followed by hydrolysis gives a ketone.
- From benzene or substituted benzene (Friedel-Craft acylation reaction)
Treatment of benzene or substituted benzene with acid chloride in presence of anhydrous aluminum chloride gives ketone.
Example :
Consider the following reaction.
The structure of the product M is
- A )
- B )
- C )
- D )
The given reaction is
Nitriles (RCN) can be reduced by DIBAL − H (Diisobutylaluminium hydride) to imine followed by hydrolysis to aldehyde. DIBAL − H does not act upon C = C and hence, prevents its reduction
Topics:Aldehydes and Ketones - Physical Properties & Chemical Reactions - I
Physical Properties of Aldehydes and Ketones
- Methanal − Gas at room temperature
- Ethanal − Volatile liquid
- Other aldehydes and ketones − Liquid or solid at room temperature
- Boling points of aldehydes and ketones are higher than those of hydrocarbons and ethers of comparable molecular masses.
- Reason: Weak molecular association in aldehydes and ketones, arising out of the dipole−dipole interactions
- Boiling points of aldehydes and ketones are lower than those of alcohols of similar molecular masses.
- Reason: Absence of intermolecular hydrogen bonding
- Lower members of aldehydes and ketones are miscible with water in all proportions.
- Reason: They form hydrogen bonds with water.
- Solubility of aldehydes and ketones decreases rapidly on increasing the length of the alkyl chain.
- All aldehydes and ketones are fairly soluble in organic solvents such as ether, methanol, etc.
- Lower aldehydes have sharp pungent odours.
- As the size of aldehydes increases, the odour becomes less pungent and more fragrant.
Chemical Reactions − I
Nucleophilic Addition Reaction
- Mechanism
- Nucleophile (Nu−) attacks the carbonyl group perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon.
- In the process, hybridisation of carbon changes from sp2 to sp3.
- A tetrahedral alkoxide is formed as intermediate.
- Reactivity
Aldehydes are more reactive than ketones in nucleophilic addition reactions.
- Reason: Steric and electronic reasons
- Examples:
- Addition of hydrogen cyanide (HCN)
- Addition of sodium hydrogen sulphite (NaHSO3)
- Addition of alcohols
- Addition of ammonia and its derivatives
Z = alkyl, aryl, OH, NH3, C6H5NH, NHCONH2, etc.
- Addition of Grignard reagents
Reduction reactions
- Reduction to alcohols
Aldehydes 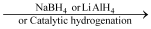 Primary alcohols
Primary alcohols
Ketones 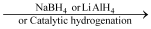 Secondary alcohols
Secondary alcohols
- Reduction to hydrocarbons
- Clemmensen reduction
- Wolf−Kishner reduction
Oxidation reactions
- Aldehydes are oxidised to carboxylic acids by common oxidising agents such as KMnO4, HNO3, K2Cr2O7, etc.
- Aldehydes are also oxidised by mild oxidising agents such as Tollen’s reagent and Fehling’s reagent. On the other hand, ketones are not oxidised by mild oxidising agents.
- Ketones are oxidised under vigorous conditions, i.e., by strong oxidising agents and at elevated temperatures. It involves carbon−carbon bond cleavage.
- Tollen’s test
- Fehling’s Test
- Oxidation of methyl ketones by haloform reaction
Example :
Consider the following reaction.
The product Z is
- A )
(C2H5) 2 C(OH) COOH
- B )
- C )
HOCH2CH(CH3) COOH
- D )
CH3 CH2 CH = CHCH2 COOH
Example :
Consider the following reaction.
The electrophilicity of the carbonyl carbon increases due to
- A )
both dry HCl gas and dilute HCl
- B )
neither dry HCl gas nor dilute HCl
- C )
only dry HCl gas
- D )
only dilute HCl
The proton, H+ of dry HCl gas protonates the oxygen of carbonyl compounds.
It increases the electrophilicity of the carbonyl carbon that enhances the chance of nucleophilic attack of ethylene glycol to give ethylene glycol ketal. Dilute HCl makes the reaction proceed in the reverse direction.
Topics:Reactions Due to α-Hydrogen & Uses of Aldehydes and Ketones
Reactions due to α-Hydrogen
- α-Hydrogen of aldehydes and ketones are acidic: They undergo a number of reactions due to the acidic nature of α-hydrogen.
- Reason for the acidity of α-hydrogen: Strong electron-withdrawing effect of the carbonyl group, and resonance stabilisation of the conjugate base
- Aldol condensation (or aldol reaction)
Aldehydes and ketones with at least one α-hydrogen undergo a reaction in the presence of dilute alkali as catalyst.
- Cross-aldol condensation: Aldol condensation carried out between two different aldehydes and/or ketones
- If both of them contain α-hydrogen atoms, then it gives a mixture of four products.
- Ketones can also be used as one component in cross-aldol reactions.
Other Reactions
- Cannizaro reaction
- Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali.
- Example:
- Electrophilic substitution reaction
- Aromatic aldehydes and ketones undergo electrophilic substitutions at the ring.
- Carbonyl group acts as a deactivating and meta-directing group.
Uses of Aldehydes and Ketones
- As solvents
- As starting materials and reagents for the synthesis of other products
- Formaldehyde {formation (40%) solution}− Used for preserving biological specimens, bakelite, urea formaldehyde glues and other polymers products
- Acetaldehyde − In the manufacture of acetic acid, ethyl acetate, vinyl acetate, polymers and drugs
- Benzaldehyde − In perfumery and in dye industries
- Butyraldehyde, vanillin, camphor, etc., are well known for their odours and flavours
- Acetone and ethyl methyl ketone − Common industrial solvents
Example :
Consider the following reaction.
What is the IUPAC name of the final product?
- A )
1, 2 − Diphenylprop − 2 − en − 1 − one
- B )
2, 2 − Diphenylprop − 2 − en − 1 − one
- C )
3, 3 − Diphenylprop − 2 − en − 1 − one
- D )
1, 3 − Diphenylprop − 2 − en − 1 − one
The reaction shown above is cross aldol condensation. In this reaction, only one reactant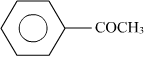 has α − hydrogen.
has α − hydrogen.
Example :
Which of the following reactions is an example of disproportionation reaction?
- A )
Wolff − Kishner reduction
- B )
Gatterman − Koch reaction
- C )
Cannizzaro reaction
- D )
Stephen reaction
Cannizzaro reaction is an example of a disproportionation reaction. In this reaction, aldehydes containing α − hydrogen atom, undergo self oxidation and reduction reaction on treatment with concentrated KOH. One molecule of the aldehyde is reduced to alcohol while another is oxidised to a salt of carboxylic acid.
Example :
Suggest the suitable procedure to prepare the following compounds from CH3—CH2CHO.
(i) CH3CH2CH2CH(CH3)CHO
(ii) CH3CH2—CH=C(CH3)CHO
(iii) CH3CH2CH(OH)CH(CH3)CO2H
(iv) CH3CH2CH2CH(CH3)CH2OH
(v) CH3CH2CH=C(CH3)CH2OH
(i)
(ii) 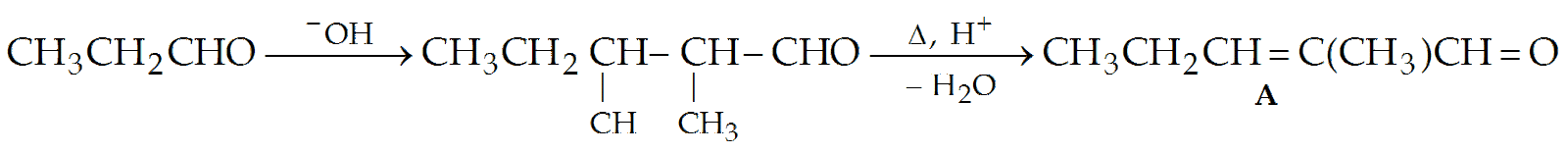
(iii) Tollen’s Reagent,  , is a specific oxidant from −CHO → CO2H
, is a specific oxidant from −CHO → CO2H
(iv) 
(v) 
Topics:Carboxyl Group - Nomenclature and Structure & Methods of Preparation
Nomenclature
In the IUPAC system, aliphatic carboxylic acids are named as follows:
- By replacing the ending ‘− e’ in the name of the corresponding alkane with ‘− oic acid’
- Carboxylic carbon is numbered one.
- If more than one carboxyl groups are present, then the ending ‘− e’ of the alkane is retained.
- The number of carboxyl groups is indicated by adding prefix, d, tri, etc. to the term ‘oic’.
The given table lists the common and IUPAC names and structures of some carboxylic acids.
Names and Structures of Some Carboxylic Acids
Structure
|
Common name
|
IUPAC name
|
HCOOH
|
Formic acid
|
Methanoic acid
|
CH3COOH
|
Acetic acid
|
Ethanoic acid
|
CH3CH2COOH
|
Propionic acid
|
Propanoic acid
|
CH3CH2CH2COOH
|
Butyric acid
|
Butanoic acid
|
(CH3)2CHCOOH
|
Isobutyric acid
|
2-Methylpropanoic acid
|
HOOC-COOH
|
Oxalic acid
|
Ethanedioic acid
|
HOOC −CH2-COOH
|
Malonic acid
|
Propanedioic acid
|
HOOC -(CH2)2-COOH
|
Succinic acid
|
Butanedioic acid
|
HOOC -(CH2)3-COOH
|
Glutaric acid
|
Pentanedioic acid
|
HOOC -(CH2)4-COOH
|
Adipic acid
|
Hexanedioic acid
|
HOOC -CH2-CH(COOH)-CH2-COOH
|
−
|
Propane-1, 2, 3-
tricarboxylic acid
|
Benzoic acid
|
Benzenecarboxylic acid
(Benzoic acid)
| |
Phenylacetic acid
|
2-Phenylethanoic acid
| |
Phthalic acid
|
Benzene-1, 2-dicarboxylic
acid
|
Structure of Carboxyl Group
- Carboxyl carbon is less electrophilic than carbonyl carbon because of resonance.
- Bonds to the carboxyl carbon lie in one plane and are separated by about 120°.
Methods of Preparation of Carboxylic Acid
- From primary alcohols
- From primary aldehydes
Oxidising agents − HNO3, KMnO4, K2Cr2O7
Mild oxidising agents − Tollen’s reagent and Fehling’s reagent
- From alkyl benzenes
- 1° and 2° alkyl benzene are oxidised in this manner.
- Tertiary group is not affected.
- From nitriles and amides
- From Grignard reagents
- From acyl halides
- From acyl anhydrides
- From esters
- Ester
Carboxylic acid
Example:
Examples:
Example :
Consider the following reaction.
The compound X is
- A )
ester
- B )
Grignard reagent
- C )
aldehyde
- D )
acyl halide
Reaction of Grignard reagent with dry ice, CO2 followed by acidification gives carboxylic acid having one carbon atom more than that present in R group of RMgX.
Example :
Consider the following reaction.
For the above reaction, R cannot be
- A )
−CH3
- B )
− CH2 CH2 CH3
- C )
- D )
−C (CH3)3
Alkyl benzene upon reaction with alk. KMnO4 followed by acidic hydrolysis gives benzoic acid. In this reaction, entire side chain is oxidised irrespective of its length. However, only primary and secondary alkyl halides are oxidised in this process while tertiary alkyl halides do not show this reaction.
−C (CH3)3 or  is tertiary, hence it is not oxidised.
is tertiary, hence it is not oxidised.
Topics:Chemical Reactions of Carboxylic Acids
Reactions Involving Cleavage of O−H Bond
Acidity
- Reactions with metals and alkalies:
- Dissociate in water to give resonance-stabilised carboxylate anions and hydronium ion
- Effects of substituents on the acidity of carboxylic acids
- The order of the effect of the groups in increasing acidity is
Ph < I < Br < Cl < F < CN < NO2 < CF3
Reactions Involving Cleavage of C−OH Bond
- Formation of anhydride
- Esterification
- Reactions with PCl5, PCl3, and SOCl2
- Reaction with ammonia
Reactions Involving −COOH group
- Reduction
- Decarboxylation
- Kolbe’s electrolysis − On electrolysis of an aqueous solution of alkali metal salts of carboxylic acids, the salts undergo decarboxylation, forming hydrocarbons containing twice the number of carbon atoms present in the alkyl group of the acid.
Substitution reactions in the hydrocarbon part
- Halogenation (Hell-Volhard-Zelinsky reaction)
- Ring substitution
- Undergo electrophilic substitution reactions (except Friedel-Craft reaction)
Uses of Carboxylic Acids
- Methanoic acid − In rubber, textile, dyeing, leather and electroplating industries
- Ethanolic acid − As a solvent and as a vinegar in food industry
- Hexanoic acid − In the manufacture of nylon-6, 6
- Higher fatty acids − For the manufacture of soaps and detergents
- Esters of benzoic acid − In perfumery
- Sodium benzoate − As a food preservative
Example :
HVZ reaction involves
- A )
reduction reaction of carboxylic group
- B )
cleavage of O − H bond of carboxylic acid
- C )
cleavage of C − OH bond of carboxylic acid
- D )
substitution reaction in hydrocarbon part of carboxylic acid
HVZ is Hell − Volhard − Zelinsky reaction. In this reaction α − hydrogen of hydrocarbon part of carboxylic acid is substituted by Cl or Br atom in presence of red phosphorus.
Example :
Consider the reaction scheme given below.
Identify the compounds A to G.
Since compound A (C4H8O3) on strong heating loses it optical activity to give compound B (C4H6O2), the former can have any one of the following structures.
Compound Bis optically inactive.

No comments:
Post a Comment