Types of Chemical Reactions
The branch of physical chemistry which deals with the rate at which the chemical reactions occur, the mechanism by which the chemical reactions take place and the influence of various factors such as concentration, temperature, pressure, catalyst etc., on the reaction rates is called the chemical kinetics.
Types of chemical reactions
On the basis of reaction rates, the chemical reactions have been classified into the following three types,
(1) Very fast or instantaneous reactions: These reactions occur at a very fast rate generally these reactions involve ionic species and known as ionic reactions. It is almost impossible to determine the rates of these reactions.
Examples
(i) 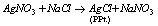 (Precipitation reaction)
(Precipitation reaction)
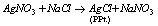 (Precipitation reaction)
(Precipitation reaction)
(ii) 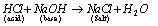 (Neutralization reaction)
(Neutralization reaction)
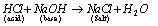 (Neutralization reaction)
(Neutralization reaction)
(2) Moderate reaction : These reactions proceed with a measurable rates at normal temperature and it is these reactions are studied in chemical kinetics. Mostly these reactions are molecular in nature.
Examples
(i) Decomposition of: H2O2 : 2H2O2 –> 2H2O + O2
(ii) Decomposition of: N2O5 : 2N2O5 –> 2N2O4 + O3
(3) Very slow reactions: These reactions are extremely slow and take months together to show any measurable change.
Examples
(i) Rusting of iron: 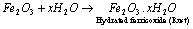
(ii) 
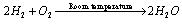
Rate of Reaction
RATE OF REACTION
The rate of a reaction is the change in concentration in per unit time.
Δx/Δt or dx/dt = (x2–x1/t2–t1)
where Δx or dx is the concentration change, i.e., (x2–x1) in the time interval t or dt, i.e., (t2–t1).
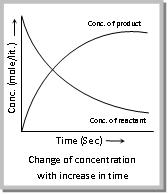
Concentration is generally expressed in active mass, i.e., mole L–1
• The rate measured over a long time interval is called average rate and the rate measured for an infinitesimally small time interval is called instantaneous rate and Instantaneous rate = (Average rate) Δt–>0
• For the reaction
Rate of disappearance of a reactant is negative
–d[A]/dt Rate of disappearance of A
–d[B]/dt Rate of disappearance of B
Rate of formation of a product is positive
d[C]/dt Rate of formation of C
d[D]/dt Rate of formation of D
• In terms of stoichiometric coefficient rate may be expressed as
dx/dt = –1/a d[A]/dt = –1/b d[B]/dt = 1/c d[C]/dt = 1/d d[D]/dt
• The rate of reaction is always positive.
• The rate of chemical reaction decreases as the reaction proceeds.
• Unit of rate of a reaction = Unit of conc./ Unit of time =mole L–1 time–1
In term of gaseous reaction the unit is atm time-1 and
Rate in atm time-1 = Rate in mole L–1 time–1 × RT
Factors affecting rate of a reaction
The rate of a chemical reaction depends on the following things
(1) Nature of reactants
(i) Physical state of reactants: This has considerable effect over rate of reaction.
Gaseous state > Liquid state > Solid state —> Decreasing rate of reaction
(ii) Physical size of the reactants: Among the solids, rate increases with decrease in particle size of the solid.
(iii) Chemical nature of the reactants
(a) Reactions involving polar and ionic substances including the proton transfer reactions are usually very fast. On the other hand, the reaction in which bonds is rearranged, or electrons transferred are slow.
(b) Oxidation-reduction reactions, which involve transfer of electrons, are also slow as compared to the ionic substance.
(c) Substitution reactions are relatively much slower.
(2) Effect of temperature: The rate of chemical reaction generally increases on increasing the temperature. The rate of a reaction becomes almost double or tripled for every rise in temperature.
Temperature coefficient of a reaction is defined as the ratio of rate constants at two temperatures differing by (generally 25°C and 35°C) 10°C.
µ = Temperture cofficient = k at (t + 10oC)/k at to C = k25oC/k35oC
(3) Concentration of reactants: The rate of a chemical reaction is directly proportional to the concentration of the reactants means rate of reaction decreases with decrease in concentration.
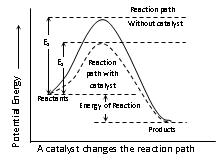
(4) Presence of catalyst: The function of a catalyst is to lower down the activation energy. The greater the decrease in the activation energy caused by the catalyst, higher will be the reaction rate.
(5) Effect of sunlight: There are many chemical reactions whose rate are influenced by radiations particularly by ultraviolet and visible light. Such reactions are called photochemical reactions. For example, Photosynthesis, Photography, Blue printing, Photochemical synthesis of compounds etc.
The radiant energy initiates the chemical reaction by supplying the necessary activation energy required for the reaction.
Solved example 1: The rate of a chemical reaction
(a) Increases as the reaction proceeds
(b) Decreases as the reaction proceeds
(c) May increase or decrease during the reaction
(d) Remains constant as the reaction proceeds
Solved example 2: The rate of a reaction that not involve gases is not dependent on
(a) Pressure (b) Temperature
(c) Concentration (d) Catalyst
Solved example 3: The rate at which a substance reacts depends on its
(a) Atomic weight (b) Equivalent weight
(c) Molecular weight (d) Active mass
Rate law
Rate law : Molecularity and Order of a reaction
Molecularity is the sum of the number of molecules of reactants involved in the balanced chemical equation. Molecularity of a complete reaction has no significance and overall kinetics of the reaction depends upon the rate determining step. Slowest step is the rate-determining step. This was proposed by Van't Hoff.
Example : NH4NO2 –> N2 + 2H2O (Unimolecular)
NO + O2 –> NO2 + O2 (Bimolecular)
2NO + O2 –> 2NO2 (Trimolecular)
The total number of molecules or atoms whose concentration determine the rate of reaction is known as order of reaction.
Order of reaction = Sum of exponents of the conc. terms in rate law
For the reaction xA + yB –> Products
The rate law is Rate = [A]x [B]y
Then the overall order of reaction. n = x + y
where x and y are the orders with respect to individual reactants.
• If reaction is in the form of reaction mechanism then the order is determined by the slowest step of mechanism.
2A + 3B –> A2B3
A + B – AB (fast)
AB + B2 –> AB3 (slow) (Rate determining step)
AB3 + A – A2B3 (fast)
(Here, the overall order of reaction is equal to two.)
• Molecularity of a reaction is derived from the mechanism of the given reaction. Molecularity cannot be greater than three because more than three molecules may not mutually collide with each other.
• Molecularity of a reaction can't be zero, negative or fractional. order of a reaction may be zero, negative, positive or in fraction and greater than three. Infinite and imaginary values are not possible.
• When one of the reactants is present in the large excess, the second order reaction conforms to the first order and is known as pesudo unimolecular reaction. (Table 11.1)
Solved example 1: Which of the following is a first order reaction
(a) NH4NO2 –> N2 + 2H2O
(b) 2HI –> H2 + I2
(c) 2NO2 –> 2NO + O2
(d) 2NO + O2 –> 2NO2
Ans: a
Solved example 2: The inversion of cane sugar is represented by
C12H22O11 + H2O –> C6H12O6 + C6H12O6
It is a reaction of
(a) Second order (b) Unimolecular
(c) Pseudo unimolecular (d) None of the three
Ans: c
Photochemical Reaction
Photochemical reaction
Absorption of radiant energy by reactant molecules brings in photophysical as well as photochemical changes. According to Einstein's law of photochemical equivalence, the basic principle of photo processes, each reactant molecule is capable of absorbing only one photon of radiant energy. The absorption of photon by a reactant molecule may lead to any of the photo process.
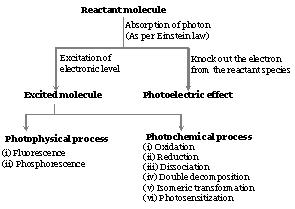
The chemical reactions, which are initiated as a result of absorption of light, are known as photochemical reactions. In such cases, the absorbed energy is sufficient to activate the reactant molecules to cross the energy barrier existing between the reactants and products or in other words, energy associated with each photon supplies activation energy to reactant molecule required for the change.
(1) Characteristics of photochemical reactions
(i) Each molecule taking part in a photo process absorbs only one photon of radiant energy thereby increasing its energy level by hv or hc/λ
(ii) Photochemical reactions do not occur in dark.
(iii) Each photochemical reaction requires a definite amount of energy which is characteristic of a particular wavelength of photon. For example, reactions needing more energy are carried out in presence of UV light (lower λ, more E/Photon). A reaction-taking place in UV light may not occur on exposure to yellow light (lower λ and lesser E/Photon)
(iv) The rate of photochemical reactions depend upon the intensity of radiation’s absorbed.
(v) The ΔG values for light initiated reactions may or may not be negative.
(vi) The temperature does not have marked effect on the rate of light initiated reactions.
(2) Mechanism of some photochemical reactions
(i) Photochemical combination of H2 and Cl2: A mixture of H2 and Cl2 on exposure to light give rise to the formation of HCl, showing a chain reaction and thereby producing 106 to 108 molecules of HCl per photon absorbed.

The mechanism leading to very high yield of HCl as a result of chemical change can be as follows. Chlorine molecules absorb radiant energy to form an excited molecule which decomposes to chlorine free radicals (Cl) to give chain initiation step.
Light absorption step : 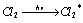 ........(i)
........(i)
 ........(i)
........(i)
Chain initiation step :  ........(ii)
........(ii)
 ........(ii)
........(ii)
The chlorine free radical then combines with H2 molecule to form HCl and H* free radical. The H* free radical so formed again combines with another Cl2 molecule to give HCl and Cl* free radical back resulting into chain propagation step.
Chain propagation step : Cl* + H2 –> HCl + H* ........(iii)
H* + Cl2 –> HCl + Cl*
The combination of two Cl* free radicals leads to chain terminating step.
Chain terminating step: CL* + Cl* –> Cl2 ........(iv)
(ii) Photochemical combination of H2 and Br2: The combination of H2 and Br2 to form HBr in presence of light is also an example of chain reaction like photochemical combination of H2 and Cl2. Here two Br2 molecules absorb photon, however, inspite of chain reaction only one molecule of HBr is formed for each 100 photon absorbed by 100 molecules of Br2.

Mechanism
Light absorption step : Br2 + Hv –> Br2* ........(i)
Chain initiation step : Br2* –> Br* + Br* ........(ii)
Chain propagation step : Br* + H2 –> HBr + H* ........(iii)
H* + Br2 –> HBr + Br* ........(iv)
Chain termination step : Br* + Br* –> Br2 ........(v)
The lower values of HBr formation per photon of light absorbed has been attributed to the fact that step (III) is highly endothermic and thus before step (III) can take place most of the bromine free radicals recombine as per step (V) to give Br2 molecule and thus providing less feasibility for step (IV) i.e. steps regenerating free radicals. Also the decomposition of HBr increases with increase in temperature.
(3) Quantum yield (or quantum efficiency) : The quantum efficiency or yield () of a photochemical reaction may be expressed as, = No. of molecules reacted or product formed/No. of photon absorbed.
(4) Application of photochemistry: Photochemistry has significant role in our daily life. Some of the photochemical reactions commonly known as cited below,
(i) Photosynthesis in plants
(ii) Photography
(iii) The formation and destruction of ozone layer
(iv) Photoetching in electronic industry
(v) Many polymerization reactions.
(vi) Modern printing technology
(vii) Free radical combinations to obtain many compounds.
Mechanism of Reaction
MECHANISM OF REACTION
(1) Reaction involving first order consecutive reactions
(i) In such reactions, the reactions form a stable intermediate compound before they are finally converted into the products.
(ii) For example, reactants (R) are first converted to intermediate (I) which is then converted to product (P) as 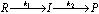
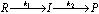
Therefore, the reaction takes place in two steps, both of which are first order i.e.,
Step I : 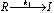 ; Step II :
; Step II : 
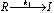 ; Step II :
; Step II : 
This means that I is produced by step I and consumed by step II. In these reactions, each stage will have its own rate and rate constant the reactant concentration will always decrease and product concentration will always increase as shown in fig.
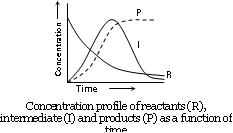
(2) Reaction involving slow step: When a reaction occurs by a sequence of steps and one of the step is slow, then the rate determining step is the slow step. For example in the reaction.
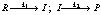 , if k1 << k2 then I is converted into products as soon as it is formed, we can say that –d[R]/dt = d[P]/dt = k1[R]
, if k1 << k2 then I is converted into products as soon as it is formed, we can say that –d[R]/dt = d[P]/dt = k1[R]
(3) Parallel reactions : In such type of reactions the reactants are more reactive, which may have different orders of the reactions taking place simultaneously. For example, in a system containing NO2 and SO2, NO2 is consumed in the following two reactions, 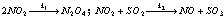
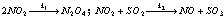
The rate of disappearance of NO2 will be sum of the rates of the two reactions i.e.,
–d[NO2]/dt = 2k1[NO2]2 + k2[NO2][SO2]
LAW OF MASS ACTION AND RATE CONSTANT
The rate at which a substance reacts is directly proportional to its active mass and the rate at which a reaction proceeds is proportional to the product of the active masses of the reacting substances.
• For a reaction, aA + bB –> product
Rate = (dx/dt) ∝ [A]a[B]b; (dx/dt) = k[A]a[B]b
Where k is called rate constant or velocity constant.
When [A] = [B] = 1 mol/litre, then dx/dt = k
Thus, rate constant k is also called specific reaction rate.
• The value of rate constant depends on, nature of reactant, temperature and catalyst. It is independent of concentration of the reactants.
• Unit of rate constant = [litre/mol]n–1 × sec–1 = [mol/litre]1–n × sec–1
Where n order of reaction.
Solved example 1: Which of these does not influence the rate of reaction
(a) Nature of the reactants
(b) Concentration of the reactants
(c) Temperature of the reaction
(d) Molecularity of the reaction
Ans: d
Solved example 2: The rate law for reaction A + 2b = C + 2D will be
(a) Rate K[A][B] (b) Rate = K[A][2B]
(c) Rate K[A][B]2 (d) Rate = K [C][D]2/[A][B]2
Ans: c
Solved example 3: In the reaction 2N2O5 –> 4NO2 + O2, initial pressure is atm and rate constant K is 3.38 × 10–5 sec–1. After 10 minutes the final pressure of N2O5 is
(a) 490 atm (b) 250 atm
(c) 480 atm (d) 420 atm
Ans: a
Arrhenius Equation
ARRHENIUS EQUATION
Arrhenius proposed a quantitative relationship between rate constant and temperature as,
k = Ae–Ea/RT …..(i)
The equation is called Arrhenius equation.
In which constant A is known as frequency factor. This factor is related to number of binary molecular collision per second per litre.
Ea is the activation energy.
T is the absolute temperature and
R is the gas constant
Both A and Ea are collectively known as Arrhenius parameters.
Taking logarithm equation (i) may be written as,
log k = log A – Ea/2.303 RT …..(ii)
The value of activation energy (Ea) increases, the value of k decreases and therefore, the reaction rate decreases.
When log k plotted against 1/T, we get a straight line. The intercept of this line is equal to log A and slope equal to –Ea/2.303R.
Therefore Ea = –2.303 R × slope.
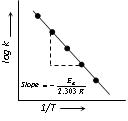
Rate constants for the reaction at two different temperatures T1 and T2,
log k2/k1 = Ea/2.303R [1/T1 – 1/T2] …..(iii)
where k1 and k2 are rate constant at temperatures T1 and T2 respectively (T1 > T2).
Solved example 1: Which one of the following does not represent Arrhenius equation
(a) k = Ae–E/RT
(b) loge k = loge A = E/RT
(c) log10 k = log10 A – E/2.303RT
(d) k = Ae–E/RT
Ans: d
Solved example 2: On increasing the temperature, the rate of the reaction increases because of
(a) Decrease in the number of collisions
(b) Decrease in the energy of activation
(c) Decrease in the number of activated molecules
(d) Increase in the number of effective collisions
Ans : d
Activation Energy
THEORIES OF REACTION RATE
(1) Collision theory
(i) The basic requirement for a reaction to occur is that the reacting species must collide with one another. This is the basis of collision theory for reactions.
(ii) The number of collisions that takes place per second per unit volume of the reaction mixture is known as collision frequency (Z). The value of collision frequency is very high of the order of 1025 to 1028 in case of binary collisions.
(iii) Every collision does not bring a chemical change. The collisions that actually produce the product are effective collisions. The effective collisions, which bring chemical change, are few in comparison to the total number of collisions. The collisions that do not form a product are ineffective elastic collisions, i.e., molecules just collide and disperse in different directions with different velocitie
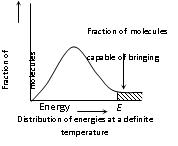
(iv) For a collision to be effective, the following two barriers are to be cleared,
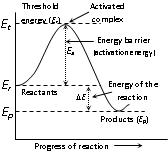
(a) Energy barrier: “The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy”.
• In the graph 'E' corresponds to minimum or threshold energy for effective collision.
• There is an energy barrier for each reaction. The reacting species must be provided with sufficient energy to cross the energy barrier.
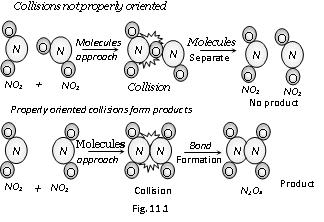
(b) Orientation barrier :The colliding molecules should also have proper orientation so that the old bonds may break and new bonds are formed. For example, NO2(g) + NO2(g) –> N2O4(g) During this reaction, the products are formed only when the colliding molecules have proper orientation at the time of collisions. These are called effective collisions.
(v) Thus, the main points of collision theory are as follows,
(a) For a reaction to occur, there must be collisions between the reacting species.
(b) Only a certain fraction of the total number of collisions is effective in forming the products.
(c) For effective collisions, the molecules should possess sufficient energy as well as orientation.
(vi) The fraction of effective collisions, under ordinary conditions may vary from nearly zero to about one for ordinary reactions. Thus, the rate of reaction is proportional to :
(a) The number of collisions per unit volume per second (Collision frequency, Z) between the reacting species
(b) The fraction of effective collisions (Properly oriented and possessing sufficient energy), f i.e., Rate = –dx/dt = f × Z
Where f is fraction of effective collision and Z is the collision frequency.
(vii) The physical meaning of the activation energy is that it is the minimum relative kinetic energy which the reactant molecules must possess for changing into the products molecules during their collision. This means that the fraction of successful collision is equal to e–Ea/RT called Boltzmann factor.
(viii) It may be noted that besides the requirement of sufficient energy, the molecules must be properly oriented in space also for a collision to be successful. Thus, if ZAB is the collision frequency, P is the orientation factor (Steric factor) then, k = PZ AB.e–Ea/RT. If we compare this equation with Arrhenius equation. k = Ae–Ea/RT
We know that pre-exponential form 'A' in Arrhenius equation is, A = PZAB.
Activation energy
The excess energy (Over and above the average energy of the reactants) which must be supplied to the reactants to undergo chemical reactions is called activation energy (Ea),
Ea = E(Timeshold energy) – E(Reactants)
Activation energy = Threshold energy – Average kinetic energy of the reacting molecules.
(a) Zero activation energy = Fraction of effective collision (f) will be very large = Very fast reaction (Instantaneous reaction).
(b) Low activation energies = Fraction of effective collision (f) will be large = Fast reactions.
(c) High activation energies = Fraction of effective collision (f) will be small = Slow reaction.
The activation energy (Ea) depends upon the nature of chemical bonds undergoing rupture and is independent of enthalpies of reactants and products.
According to the concept of activation energy, the reactants do not change directly into the products. The reactant first absorb energy equal to activation energy and form activated complex. At this state, the molecules must have energy at least equal to the threshold energy. This means that the reaction involves some energy barrier which must be overcome before products are formed. The energy barrier is known as activation energy barrier.
(2) Transition state theory
(i) According to transition state theory the activated complex is supposed to be in equilibrium with the reactant molecules.
(ii) Once the transition state is formed it can either return to the initial reactants or proceeds to form the products.
(iii) Assuming that once formed the transition state proceeds to products we can say that rate is proportional to concentration of transition state.
Mathematically, Rate ∝Transition state
Rate= Constant × Transition state
(iv) The activation energy for the forward reaction, (Efa)and the activation energy for the reverse reaction (Era) are related to the enthalpy (ΔH) of the reaction by the equation ΔH = Efa – Era.
(a) For endothermic reactions, Δ H > 0, so that Era < Efa
(b) For exothermic reaction, ΔH < 0, so that Era < Efa
1. A large increase in the rate of a reaction for a rise in temperature is due to
(a) The decrease in the number of collisions
(b) The increase in the number of activated molecules
(c) The shortening of the mean free path
(d) The lowering of the activation energy
Ans: b
2. Which of the following statements is not true according to collision theory of reaction rates
(a) Collision of molecules is a precondition for any reaction to occur
(b) All collisions result in the formation of the products
(c) Only activated collisions result in the formation of the products
(d) Molecules which have acquired the energy of activation can collide effectively
Ans: b
3. According to the collision theory of chemical reactions
(a) A chemical reaction occurs with every molecular collision
(b) Rate is directly proportional to the number of collisions per second
(c) Reactions in the gas phase are always of zero order
(d) Reaction rates are of the order of molecular speeds
Ans: b

No comments:
Post a Comment