d-Block elements
A transition element may be defined as an element whose atom in the ground state or ion in common oxidation state has incomplete sub-shell, has electron 1 to 9. It is called transition element due to fact that it is lying between most electropositive (s-block) and most electronegative (p-block) elements and represent a transition from them. The general electronic configuration of these element is (n–1)1 to 10 ns 0 to 2
The definition of transition metal excludes Zn, Cd and Hg because they have complete d- orbital. Their common oxidation state is Zn++, Cd++, Hg++ They also do not show the characteristics of transition element. Element of group 3 (Sc, Y, La and Ac) and group 12 (Zn, Cd, Hg) are called non typical transition element.
First transition or 3d series :
Element
|
Symbol
|
At. No.
|
Electronic configuration
| |
Scandium
|
Sc
|
21
|
3d-orbitals are filled up
|
[Ar] 3d14s2
|
Titanium
|
Ti
|
22
|
[Ar] 3d24s2
| |
Vanadium
|
V
|
23
|
[Ar] 3d34s2
| |
Chromium
|
Cr*
|
24
|
[Ar] 3d54s1
| |
Manganese
|
Mn
|
25
|
[Ar] 3d54s2
| |
Iron
|
Fe
|
26
|
[Ar] 3d64s2
| |
Cobalt
|
Co
|
27
|
[Ar] 3d74s2
| |
Nickel
|
Ni
|
28
|
[Ar] 3d84s2
| |
Copper
|
Cu*
|
29
|
[Ar] 3d104s1
| |
Zinc
|
Zn
|
30
|
[Ar] 3d104s2
| |
Second transition or 4d-series :
Element
|
Symbol
|
At. No.
|
Electronic configuration
| |
Yttrium
|
Y
|
39
|
4d-orbitals are filled up
|
[Kr] 4d15s2
|
Zirconium
|
Zr
|
40
|
[Kr] 4d25s2
| |
Niobium
|
Nb*
|
41
|
[Kr] 4d45s1
| |
Molybdenum
|
Mo*
|
42
|
[Kr] 4d55s1
| |
Technetium
|
Tc
|
43
|
[Kr] 4d55s2
| |
Ruthenium
|
Ru*
|
44
|
[Kr] 4d75s1
| |
Rhodium
|
Rh*
|
45
|
[Kr] 4d85s1
| |
Palladium
|
Pd*
|
46
|
[Kr] 4d105s0
| |
Silver
|
Ag*
|
47
|
[Kr] 4d105s1
| |
Cadmium
|
Cd
|
48
|
[Kr] 4d105s2
| |
Third transition or 5d-series :
Element
|
Symbol
|
At. No.
|
Electronic configuration
| |
Lanthanum
|
La
|
57
|
5d-orbitals are filled up
|
[Xe] 5d16s2
|
Hafnium
|
Hf
|
72
|
[Xe] 4f145d26s2
| |
Tantalum
|
Ta
|
73
|
[Xe] 4f145d36s2
| |
Tungsten
|
W
|
74
|
[Xe] 4f145d46s2
| |
Rhenium
|
Re
|
75
|
[Xe] 4f145d56s2
| |
Osmium
|
Os
|
76
|
[Xe] 4f145d66s2
| |
Iridium
|
Ir
|
77
|
[Xe] 4f145d76s2
| |
Platinum
|
Pt*
|
78
|
[Xe] 4f145d106s0
| |
Gold
|
Au*
|
79
|
[Xe] 4f145d106s!
| |
Mercury
|
Hg
|
80
|
[Xe] 4f145d106s
| |
Fourth transition or 6d-series :
Element
|
Symbol
|
At. No.
|
Electronic configuration
| |
Actinium
|
Ac
|
89
|
6d-orbitals are filled up
|
[Rn] 6d17s2
|
Rutherfordium
|
Rf
|
104
|
[Rn] 5f146d27s2
| |
Hahnium
|
Ha
|
105
|
[Rn] 5f146d37s2
| |
Seaborgium
|
Sg
|
106
|
[Rn] 5f146d47s2
| |
Bohrium
|
Bh
|
107
|
[Rn] 5f146d57s2
| |
Hassium
|
Hs
|
108
|
[Rn] 5f146d67s2
| |
Meitnerium
|
Mt
|
109
|
[Rn] 5f146d77s2
| |
Ununnilium
|
Uun
|
110
|
[Rn] 5f146d87s2
| |
Unununium
|
Uuu
|
111
|
[Rn] 5f146d97s2
| |
Unubium
|
Uub
|
112
|
[Rn] 5f146d107s2
| |
Elements marked with asterisk have anomalous configurations. These are attributed to factors like nuclear-electron and electron-electron forces and stability of half filled and full filled orbital.
All transition elements are d block elements but all d block elements are not transition elements.
Atomic Radii of D Block Element
Atomic radii
The atomic, radii of 3d-series of elements are compared with those of the neighbouring s and p-block elements.
K
Ca
|
Sc
|
Ti
|
V
|
Cr
|
Mn
| |
227
|
197
|
144
|
132
|
122
|
117
|
117
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
Ga
|
Ge
|
117
|
116
|
115
|
117
|
125
|
135
|
122*
|
* in pm units
The atomic radii of transition elements show the following characteristics,
(i) The atomic radii and atomic volumes of d-block elements in any series decrease with increase in the atomic number. The decrease however, is not regular. The atomic radii tend to reach minimum near at the middle of the series, and increase slightly towards the end of the series.
Explanation: When we go in any transition series from left, to right, the nuclear charge increases gradually by one unit at each elements. The added electrons enter the same penultimate shell, (inner d- shell). These added electrons shield the outermost electrons from the attraction of the nuclear charge. The increased nuclear charge tends to reduce the atomic radii, while the added electrons tend to increase the atomic radii. At the beginning of the series, due to smaller number of electrons in the d-orbitals, the effect of increased nuclear charge predominates, and the atomic radii decrease. Later in the series, when the number of d-electrons increases, the increased shielding effect and the increased repulsion between the electrons tend to increase the atomic radii. Somewhere in the middle of the series, therefore the atomic radii tend to have a minimum value as observed.
(ii) The atomic radii increase while going down in each group. However, in the third transition series from hafnium (Hf) and onwards, the elements have atomic radii nearly equal to those of the second transition elements.
Explanation: The atomic radii increase while going down the group. This is due to the introduction of an additional shell at each new element down the group. Nearly equal radii of second and third transition series elements is due to a special effect called lanthanide contraction.
1. The atomic radii of the elements are almost same of which series
(a) Fe – Co – Ni (b) Na – K – Rb
(c) F – Cl – Br (d) Li – Be – B
Ans: c
2. A reduction in atomic size with increase in atomic number is a characteristic of elements of
(a) High atomic masses (b) d-block
(c) f –block (d) Radioactive series
Ans: a
Actinides
Actinides
The elements with atomic numbers 90 to 103 i.e. thorium to lawrencium (which come immediately after actinium, Z = 89) are called actinides or actinones. These elements involve the filling of 5 f-orbitals. Their general electronic configuration is, [ Rn ]5 f1-14 6d0-1 7s2.
They include three naturally occuring elements thorium, protactinium and uranium and eleven transuranium elements or transuranics which are produced artificially by nuclear reactions. They are synthetic or man made elements. All actinides are radioactive.
Properties of actinides
(i) Oxidation state: The dominant oxidation state of actinides is +3 which shows increasing stability for the heavier elements. Np shows +7 oxidation state but this is oxidising and is reduced to the most stable state +5. Pu also shows states upto +7 and Am upto +6 but the most stable state drops to Pu (+4) and Am (+3). Bk in +4 state is strongly oxidising but is more stable than Cm and Am in 4 state due to f 7 configuration. Similarly, No is markedly stable in +2 state due to its f14 configuration. When the oxidation number increases to + 6, the actinide ions are no longer simple. The high charge density causes the formation of oxygenated ions e.g., UO2+2 , NpO2+2 etc. The exhibition of large number of oxidation states of actinides is due to the fact that there is a very small energy gap between 5f, 6d and 7s subshells and thus all their electrons can take part in bond formation.
(ii) Actinide contraction: There is a regular decrease in ionic radii with increase in atomic number from Th to Lr. This is called actinide contraction analogous to the lanthanide contraction. It is caused due to imperfect shielding of one 5f electron by another in the same shell. This results in increase in the effective nuclear charge which causes contraction in size of the electron cloud.
(iii) Colour of the ions: Ions of actinides are generally coloured which is due to f – f transitions. It depends upon the number of electrons in 5 f orbitals.
(iv) Magnetic properties: Like lanthanides, actinide elements are strongly paramagnetic. The magnetic moments are lesser than the theoretically predicted values. This is due to the fact that 5f electrons of actinides are less effectively shielded which results in quenching of orbital contribution.
(v) Complex formation: Actinides have a greater tendency to form complexes because of higher nuclear charge and smaller size of their atoms. They form complexes even with p-bonding ligands such as alkyl phosphines, thioethers etc, besides EDTA, β-diketones, oxine etc. The degree of complex formation decreases in the order.
M4+> MO2+2 > M3 > MO+2
Where M is element of actinide series. There is a high concentration of charge on the metal atom inMO2+2 which imparts to it relatively high tendency towards complex formation.
Oxidation states
Most of the transition elements exhibit several oxidation states i.e., they show variable valency in their compounds. Some common oxidation states of the first transition series elements are given below in table,
Outer Ele. Confi. and O. S. for 3d- elements
Elements
Outer electronic configuration
Oxidation states
Sc
3d1 4s2
+ 2, + 3
Ti
3d2 4s2
+ 2, + 3, + 4
V
3d3 4s2
+ 2,+ 3,+ 4,+ 5
Cr
3d5 4s1
+ 1, + 2, + 3, + 4, + 5, + 6
Mn
3d54s2
+ 2, + 3, + 4, + 5, + 6, + 7
Fe
3d64s2
+ 2, + 3, + 4, + 5, + 6
Co
3d74s2
+ 2, + 3, + 4
Ni
3d84s2
+ 2, + 3, + 4
Cu
3d104s1
+ 1,+ 2
Zn
3d104s2
+ 2
The relative stability of the different oxidation states depends upon the factors such as, electronic configuration, nature of bonding, stoichiometry, lattice energies and solvation energies. The highest oxidation states are found in fluorides and oxides because fluorine and oxygen are the most electronegative elements. The highest oxidation state shown by any transition metal is eight. The oxidation state of eight is shown by Ru and Os.
An examination of the common oxidation states reveals the following conclusions.
(i) The variable oxidation states shown by the transition elements are due to the participation of outer ns and inner (n–1)d-electrons in bonding.
(ii) Except scandium, the most common oxidation state shown by the elements of first transition series is +2. This oxidation state arises from the loss of two 4s electrons. This means that after scandium, d-orbitals become more stable than the s-orbital.
(iii) The highest oxidation states are observed in fluorides and oxides. The highest oxidation state shown by any transition elements (by Ru and Os) is 8.
(iv) The transition elements in the + 2 and + 3 oxidation states mostly form ionic bonds. In compounds of the higher oxidation states (compound formed with fluorine or oxygen), the bonds are essentially covalent. For example, in permanganate ion MnO4–, all bonds formed between manganese and oxygen are covalent.
(v) Within a group, the maximum oxidation state increases with atomic number. For example, iron shown the common oxidation state of + 2 and + 3, but ruthenium and osmium in the same group form compounds in the + 4, + 6 and + 8 oxidation states.
(vi) Transition metals also form compounds in low oxidation states such as +1 and 0. For example, nickle in, nickel tetracarbonyl, Ni(CO)4 has zero oxidation state. Similarly Fe in Fe(CO)5 has zero oxidation state.
The bonding in the compounds of transition metals in low oxidation states is not always very simple.
Example 1: The highest oxidation state of Cr will be
(a) 2 (b) 3
(c) 4 (d) 6
Ans: d
Elements
|
Outer electronic configuration
|
Oxidation states
|
Sc
|
3d1 4s2
|
+ 2, + 3
|
Ti
|
3d2 4s2
|
+ 2, + 3, + 4
|
V
|
3d3 4s2
|
+ 2,+ 3,+ 4,+ 5
|
Cr
|
3d5 4s1
|
+ 1, + 2, + 3, + 4, + 5, + 6
|
Mn
|
3d54s2
|
+ 2, + 3, + 4, + 5, + 6, + 7
|
Fe
|
3d64s2
|
+ 2, + 3, + 4, + 5, + 6
|
Co
|
3d74s2
|
+ 2, + 3, + 4
|
Ni
|
3d84s2
|
+ 2, + 3, + 4
|
Cu
|
3d104s1
|
+ 1,+ 2
|
Zn
|
3d104s2
|
+ 2
|
Catalytic Properties of D block Elements
Catalytic properties
Most of the transition metals and their compounds particularly oxides have good catalytic properties. Platinum, iron, vanadium pentoxide, nickel, etc., are important catalysts. Platinum is a general catalyst. Nickel powder is a good catalyst for hydrogenation of unsaturated organic compound such as, hydrogenation of oils some typical industrial catalysts are,
(i) Vanadium pentoxide (V2O5) is used in the Contact process for the manufacture of sulphuric acid,
(ii) Finely divided iron is used in the Haber’s process for the synthesis of ammonia.
Explanation : Most transition elements act as good catalyst because of,
(i) The presence of vacant d-orbitals.
(ii) The tendency to exhibit variable oxidation states.
(iii) The tendency to form reaction intermediates with reactants.
(iv) The presence of defects in their crystal lattices.
Alloy formation : Transition metals form alloys among themselves. The alloys of transition metals are hard and high metals are high melting as compared to the host metal. Various steels are alloys of iron with metals such as chromium, vanadium, molybdenum, tungsten, manganese etc.
Explanation : The atomic radii of the transition elements in any series are not much different from each other. As a result, they can very easily replace each other in the lattice and form solid solutions over an appreciable composition range. Such solid solutions are called alloys.
Chemical reactivity : The d-block elements (transition elements) have lesser tendency to react, i.e., these are less reactive as compared to s-block elements.
Explanation : Low reactivity of transition elements is due to,
(i) Their high ionisation energies.
(ii) Low heats of hydration of their ions.
(iii) Their high heats of sublimation.
Example 1: The catalytic activity of the transition metals and their compounds is ascribed to their
(a) Chemical reactivity
(b) Magnetic behaviour
(c) Unfilled d-orbitals
(d) Ability to adopt multiple oxidation states and their complexing ability
Ans: d
Example 2: Which one of the following statement is true for transition elements
(a) They exhibit diamagnetism
(b) They exhibit inert pair effect
(c) They do not form alloys
(d) They show variable oxidation states
Ans: d
Chemical Properties of Potassium Dichromate
Chemical properties Of Potassium Dichromate
(i) Action of heat: Potassium dichromate when heated strongly. Decomposes to give oxygen.
?
4K2Cr2O7(s) ————→ 4K2CrO4(s) + 2Cr2O3(s) + 3O2
(ii) Action of acids
(a) In cold, with concentrated H2SO4, red crystals of chromium trioxide separate out.
K2Cr2O7(aq) + conc.H2SO4 → KHSO4(aq) + 2CrO3(s) + H2O
On heating a dichromate-sulphuric acid mixture, oxygen gas is given out.
2K2Cr2O7 + 8H2SO4 → 2K2SO4 + 2Cr2(SO4)3 + 8H2O + 3O2
(b) With HCl, on heating chromic chloride is formed and Cl2 is liberated.
K2Cr2O7(aq) + 14HCl(aq) → 2CrCl3(aq) + 2KCl(aq) + 7H2O + 3Cl2(g)
(iii) Action of alkalies: With alkalies, it gives chromates. For example, with KOH,
On acidifying, the color again changes to orange-red owing to the formation of dichromate.
2K2CrO4 + H2SO4 → K2Cr2O7 + K2SO4 + H2O
Actually, in dichromate solution, the ions are in equilibrium with ions.
Cr2O72– + H2O 2CrO42– + 2H+
(iv) Oxidizing nature : In neutral or in acidic solution, potassium dichromate acts as an excellent oxidizing agent, and Cr2O72–gets reduced to Cr3+. The standard electrode potential for the reaction,
Cr2O72– + 14H+ + 6e– → 2Cr+3 + 7H2O is + 1.31V.
This indicates that dichromate ion is a fairly strong oxidizing agent, especially in strongly acidic solutions. That is why potassium dichromate is widely used as an oxidizing agent, for quantitative estimation of the reducing agents such as, Fe2+. It oxidizes,
(a) Ferrous salts to ferric salts
K2CrO7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
2FeSO4 + H2SO4 + [O] → Fe2[SO4]3 + H2O × 3
K2Cr2O7 + 6FeSO4 + 7H2SO4 → K2SO4 + Cr2(SO4)3 + 3Fe2(SO4)3 + 7H2O
Ionic equation
Cr2O72– + 14H+ + 6Fe2+ → 2Cr3+ + 6Fe3+ + 7H2O
(b) Sulphites to sulphates and arsenites to arsenates.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
Na2SO3 + [O] → Na2SO4] × 3
K2Cr2O7 + 4H2SO4 + 3Na2SO3 → K2SO4 + Cr2(SO4)3 + 3Na2SO4 + 4H2O
Ionic equation
Cr2O72– + 8H+ + 3SO32– → 2Cr3+ + 3SO42– + 4H2O
Similarly, arsenites are oxidised to arsenates.
Cr2O72– + 8H+ + 3AsO33– → 2Cr3+ + 3AsO43– + 4H2O
(c) Hydrogen halides to halogens.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
2HX + O → H2O + X2] × 3
K2Cr2O7 + 4H2SO4 + 6HX → K2SO4 + Cr2(SO4)3 + 7H2O + 3X2
where, X may be Cl, Br, I.
Cr2O72– + 8H+ + 6HX → 2Cr3+ + 3X2 + 7H2O
(d) Iodides to iodine
K2Cr2O7 + H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
2KI + H2O + [O] → 2KOH + I2] × 3
K2Cr2O7 + 7H2SO4 + 6KI → 4K2SO4 + Cr2(SO4)3 + 3I2 + 7H2O
Ionic equation :
Cr2O72– + 14H+ + 6I– → 2Cr3+ + 7H2O + 3I2
Thus, when KI is added to an acidified solution of K2Cr2O7 iodine gets liberated.
(e) It oxidizes H2S to S.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O]
H2S + [O] → H2O + S] × 3
K2Cr2O7 + 4H2SO4 + 3H2S → K2SO4 + Cr2(SO4)3 + 7H2O + 3S
Ionic equation
Cr2O72– + 8H+ + H2S → 2Cr3+ + 3S + 7H2O
(v) Formation of insoluble chromates : With soluble salts of lead, barium etc., potassium dichromate gives insoluble chromates. Lead chromate is an important yellow pigment.
2Pb(NO3)2 + K2Cr2O7 + H2O → 2PbCrO4 + 2KNO3 + 2HNO3
(vi) Chromyl chloride test : When potassium dichromate is heated with conc. H2SO4 in the presence of a soluble chloride salt, the orange-red vapors of chromyl chloride (CrO2Cl2) are formed.
heat
K2Cr2O7 + 4NaCl + 6H2SO4 ————→ 2KHSO4 + 4NaHSO3 + 2CrO2Cl2
Chromyl chloride vapors when passed through water give yellow-colored solution containing chromic acid.
CrO2Cl2 + 2H2O → 2HCl + H2CrO4 Chromic acid (yellow solution)
Chromyl chloride test can be used for the detection of chloride ion is any mixture.
Uses : Potassium dichromate is used as,
(i) An oxidizing agent
(ii) In chrome tanning
(iii) The raw material for preparing large number of chromium compounds
(iv) Primary standard in the volumetric analysis.
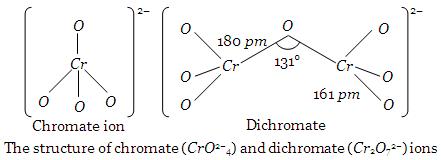
Structures of Chromate and Dichromate Ions
Chromates and dichromate are the salts of chromic acid (H2CrO4). In solution, these ions exist in equilibrium with each other. Chromate ion has four oxygen atoms arranged tetrahedrally around Cr atom. (see Fig). Dichromate ion involves a Cr–O–Cr bond as shown in Fig.
Chemical Properties of Potassium Permanganate
Chemical properties of Potassium Permanganate
Some important chemical reactions of KMnO4 are given below,
Action of heat: KMnO4 is stable at room temperature, but decomposes to give oxygen at higher temperatures.
heat
2KMnO4(s) ————→ K2MnO4(s) + MnO2 + O2(g)
Oxidizing actions : KMnO4 is a powerful agent in neutral, acidic and alkaline media. The nature of reaction is different in each medium. The oxidising character of KMnO4 (to be more specific, of MnO4–) is indicated by high positive reduction potentials for the following reactions.
Acidic medium:
MnO4– + 8H+ + 5e– ? Mn2+ + 4H2O Eo = 1.51 V
Alkaline medium
MnO4– + 2H2O + 3e– ? MnO2 + 4OH– Eo = 1.23 V
In strongly alkaline solutions and with excess of MnO4–, the reaction is
MnO4– + e– ? MnO42– Eo = 0.56 V
There are a large number of oxidation-reduction reactions involved in the chemistry of manganese compounds. Some typical reactions are,
In the presence of excess of reducing agent in acidic solutions permanganate ion gets reduced to manganous ion, e.g.,
5Fe2+ + MnO4– + 8H+ → 5Fe3+ ? Mn2+ + 4H2O
An excess of reducing agent in alkaline solution reduces permanganate ion only to manganese dioxide e.g.,
3NO2– + MnO4– + 2OH– → 3NO3– + MnO2 + 4H2O
In faintly acidic and neutral solutions, manganous ion is oxidized to manganese oxidized to manganese dioxide by permanganate.
2MnO4– + 3Mn+2 + 2H2O → 5MnO2 + 4H+
In strongly basic solutions, permangante oxidizes manganese dioxide to manganate ion.
MnO2 + 2MnO4– + 4OH– → 3MnO42– + 2H2O
In acidic medium, KMnO4 oxidizes,
Ferrous salts to ferric salts
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
2FeSO4 + H2SO4 + [O] → Fe2(SO4)3 + H2O] × 5
2KMnO4 + 8H2SO4 + 10FeSO4 → K2SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H2O
Ionic equation
2MnO4– + 16H+ + 10Fe2+ → 2Mn2+ + 10Fe3+ + 8H2O
The reaction forms the basis of volumetric estimation of Fe2+ in any solution by KMnO4.
Oxalic acid to carbon dioxide
2KmNO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
(COOH)2 + [O] → 2CO2 + H2O] × 3
2KMnO4 + 3H2SO4 + 5(COOH)2 → K2SO4 + 2MnSO4 + 10CO2 + 8H2O
Ionic equation
2MnO4– + 6H+ + 5(COOH)2 → 2Mn2+ + 10CO2 + 8H2O
Sulphites to sulphates
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
Na2SO3 + [O] → Na2SO4] × 5
2KMnO4 + 3H2SO4 + 5Na2SO3 → K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O
Ionic equation
2MnO4– + 6H+ + 5SO32– → 2Mn2+ + 5SO42– + 3H2O
Iodides to iodine in acidic medium
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
2KI + H2O + [O] → I2 + 2KOH × 5
2KOH + H2SO4 → K2SO4 + 2H2O] × 5
2KMnO4 + 8H2SO4 + 10 KI → 6K2SO4 + 2MnSO4 + 5I2 + 8H2O
Ionic equation
2MnO4– + 16H+ + 10I– → 2Mn2+ + 5I2 + 8H2O
Hydrogen peroxide to oxygen
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
H2O2 + [O] → H2O + O2 × 5
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2
Manganous sulphate (MnSO4) to manganese dioxide (MnO2)
2KMnO4 + H2O → 2KOH + 2MnO2 + 3[O]
MnSO4 + H2O + [O] → MnO2 + H2SO4 × 3
2KOH + H2SO4 → K2SO4 + 2H2O
2KMnO4 + 3MnSO4 + 2H2O → 5MnO2 + K2SO4 + 2H2SO4
Ionic equation
2MnO4– + 3Mn2+ + 2H2O → 5MnO2 + 4H+
Ammonia to nitrogen
2KMnO4 + H2O → 2MnO2 + 2KOH + 3[O]
2NH3 + 3[O] → N2(g) + 3H2O
2KMnO4 + 2NH3 → 2MnO2 + 2KOH + 2H2O + N2(g)
Uses : KMnO4 is used,
(i) As an oxidizing agent. (ii) As a disinfectant against disease-causing germs. (iii) For sterilizing wells of drinking water. (iv) In volumetric estimation of ferrous salts, oxalic acid etc. (v) Dilute alkaline KMnO4 solution known as Baeyer’s reagent.
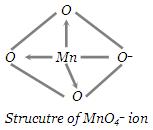
Structure of Permanganate Ion (MnO4–) : Mn in MnO4– is in +7 oxidation state. Mn7+ exhibits sp3hybridisation in this ion. The structure of MnO4– is, shown in fig.

Chromium containing Compounds
Chromium Containing Compounds
Potassium dichromate, (K2Cr2O7)
Potassium dichromate is one of the most important compound of chromium, and also among dichromates. In this compound Cr is in the hexavalent (+6) state.
Preparation : It can be prepared by any of the following methods,
(i) From potassium chromate : Potassium dichromate can be obtained by adding a calculated amount of sulphuric acid to a saturated solution of potassium chromate.
2K2CrO4 + H2SO4 → K2Cr2O7 + K2SO4 + H2O
potassium chromate potassium dichromate
(yellow) (orange)
K2Cr2O7 Crystals can be obtained by concentrating the solution and crystallisation.
(ii) Manufacture from chromite ore : K2Cr2O7 is generally manufactured from chromite ore (FeCr2O4). The process involves the following steps.
(a) Preparation of sodium chromate : Finely powdered chromite ore is mixed with soda ash and quicklime. The mixture is then roasted in a reverberatory furnace in the presence of air. Yellow mass due to the formation of sodium chromate is obtained.
4FeCr2O4 + O2 → 2FeO3 + 4Cr2O3
4FeCr2O4 + 8Na2CO3 + 6O2 → 8Na2CrO4 + 8CO2(g)
4FeCr2O4 + 8Na2CO3 + 7O2 → 2Fe2O3 + 8CO2(g) + 8Na2CrO4
sodium chromate
The yellow mass is extracted with water, and filtered. The filtrate contains sodium chromate.
The reaction may also be carried out by using NaOH instead of Na2CO3. The reaction in that case is,
4FeCr2O4 + 16NaOH + 7O2 → 8Na2CrO4 + 2FeO3 + 8H2O
(b) Conversion of chromate into dichromate : Sodium chromate solution obtained in step (a) is treated with concentrated sulphuric acid when it is converted into sodium dichromate.
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
sodium chromate sodium dichromate
On concentration, the less soluble sodium sulphate, Na2SO4.10H2O crystallizes out. This is filtered hot and allowed to cool when sodium dichromate, Na2Cr2O7.2H2O, separates out on standing.
(c) Concentration of sodium dichromate to potassium dichromate : Hot concentrated solution of sodium dichromate is treated with a calculated amount of potassium chloride. When potassium dichromate being less soluble crystallizes out on cooling.
Na2Cr2O7 + 2KCl → KrCr2O7 + 2NaCl
sod. dichromate pot. dichromate
Physical properties
(i) Potassium dichromate forms orange-red coloured crystals.
(ii) It melts at 699 K.
(iii) It is very stable in air (near room temperature) and is generally, used as a primary standard in the volumetric analysis.
(iv) It is soluble in water though the solubility is limited.
Compounds of Copper
Compounds of Copper
(1) Halides of copper : Copper (II) chloride, CuCl2 is prepared by passing chlorine over heated copper. Concentrated aqueous solution of CuCl2 is dark brown but changes first to green and then to blue on dilution.
On heating, it disproportionates to copper (I) chloride and chlorine
Heat
2CuCl2 ————→ 2CuCl + Cl2
It is used as a catalyst in the Daecon’s process for the manufacture of chlorine.
Copper (I) chloride, CuCl is a white solid insoluble in water. It is obtained by boiling a solution of CuCl2 with excess of copper turnings and conc. HCl.
HCl
CuCl2 + Cu ————→ 2CuCl
It dissolves in conc. HCl due to the formation of complex H[CuCl2]
CuCl + HCl ————→ H [CuCl2]
It is used as a catalyst alongwith NH4Cl in the preparation of synthetic rubber.
(2) Cuprous oxide Cu2O: It is a reddish brown powder insoluble in water but soluble in ammonia solution, where it forms diammine copper (I) ion. Cu+ + 2NH3 —→ [Cu(NH3)2]+. It is used to impart red colour to glass in glass industry.
(3) Cupric oxide CuO: It is dark black, hygroscopic powder which is reduced to Cu by hydrogen, CO etc. It is used to impart light blue colour to glass. It is prepared by heating copper nitrate.
?
2Cu(NO3)2 ————→ 2CuO + 4NO2 + O2
(4) Copper sulphate CuSO4.5H2O (Blue vitriol) : It is prepared by action of dil H2SO4 on copper scrap in presence of air.
2Cu + 2H2SO4 + O2 —→ CuSO4 + 2H2O
(air)
(i) On heating this blue salt becomes white due to loss of water of crystallization.
503K
CuSO4.5H2O ————→ CuSO4 + 5H2O
Blue White
At about 1000 K, CuSO4 decomposes to give CuO and SO3.
1000K
CuSO4 ————→ CuO + SO3
(ii) It gives a deep blue solution of tetrammine copper (II) sulphate with
Cu2SO4 + 4NH4OH, ————→ [Cu(NH3)4]SO4 + 4H2O
Blue colour
(iii) With KCN it first gives yellow precipitate of CuCN which decomposes of give Cu2(CN)2.Cu2(CN)2dissolves in excess of KCN to give K3[Cu(CN)4]
(iv) With KI it gives white ppt. of Cu2I2
4KI + 2CuSO4 → 2K2SO4 + Cu2I2 + I2
white ppt.
(v) With K4[Fe(CN)6], CuSO4 gives a reddish brown ppt. of Cu2[Fe(CN)6]
2CuSO4 + K4[Fe(CN)6] → Cu2[Fe(CN)6] + 2K2SO4
Reddish brown ppt.
Uses: For electroplating and electrorefining of copper. As a mordant in dyeing. For making Bordeaux mixture (11 parts lime as milk of lime + 16 parts copper sulphate in 1,000 parts of water). It is an excellent fungicide. For making green pigments containing copper carbonate and other compounds of copper. Like Verdigris which is Cu(CH3COO)2Cu(OH)2 i.e. basic copper acetate and is used as a green pigment in paints. As a fungicide in starch paste for book binding work.
(5) Cupric sulphide CuS It is prepared as follows : Cu(NO3)2 + H2S → CuS + 2HNO3.
Black ppt.
(6) Basic copper carbonates : Because of lower solubility of the hydroxide, the normal carbonate does not exist. Two basic copper carbonates occur in nature viz malachite CuCO3.Cu(OH)2 which has a fine green colour, and azurite, 2CuCO3, Cu(OH)2 which is deep blue in colour.
Malachite is prepared by heating a mixture of CuSO4 solution and limestone in a sealed tube at 423 – 443 K
423-443 K
2CuSO4 + 2CaCO3 + H2O —————→ CuCO3Cu(OH)2 + 2CaSO4 + CO2
Malachite
At lower temperature azurite is formed
< 423
3CuSO4 + 3CaCO3 + H2O —————→ 2CuCO3Cu(OH)2 + 3CaSO4 + CO2
Azurite
On heating, both decompose to give black cupric oxide, water and CO2.
They are used as green and blue painter’s pigments under the name ‘malachite green’ and azurite blue’.
Compounds of Iron
Compounds of iron
(1) Oxides of Iron : Iron forms three oxides FeO, Fe2O3 (Haematite), Fe3O4 (magnetite also called magnetic oxide or load stone).
(i) Ferrous oxide, FeO: It is a black powder, basic in nature and reacts with dilute acids to give ferrous salts.
FeO + H2SO4 → FeSO4 + H2O; It is used in glass industry to impart green colour to glass.
(ii) Ferric oxide Fe2O3: It is a reddish brown powder, not affected by air or water; amphoteric in nature and reacts both with acids and alkalis giving salts. It can be reduced to iron by heating with C or CO.
Fe2O3 + 3C → 2Fe + 3CO; Fe2O3 + 3CO → 2Fe + 3CO2
It is used as red pigment to impart red colour to external walls and as a polishing powder by jewellers.
(iii) Ferroso ferricoxide Fe3O4(FeO.Fe2O3): It is more stable than FeO and Fe2O3 magnetic in nature and dissolves in acids giving a mixture of iron (II) and iron (III) salts.
Fe3O4 + 4H2SO4 (dil.) → FeSO4 + Fe2(SO4)3 + 4H2O
(2) Ferrous sulphide FeS: It is prepared by heating iron filing with sulfur. With dilute H2SO4 it gives H2S.FeS + H2SO4 (dil) → FeSO4 + H2S ↑
(3) Ferric chloride FeCl3: (i) preparation: It is prepared by Fe(OH)3 treating with HCl
Fe(OH)3 + 3HCl → FeCl3 + 3H2O
The solution on evaporation give yellow crystals of FeCl3.6H2O
(ii) Properties: (a) Anhydrous FeCl3 forms reddish-black deliquescent crystals.
(b) FeCl3 is hygroscopic and dissolves in H2O giving brown acidic solution due to formation of HCl
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
(Brown)
(c) Due to oxidising nature Fe3+ ions FeCl3 is used in etching metals such as copper
2Fe3+ + Cu → 2Fe2+ + Cu2+ (aq)
(d) In vapour state FeCl3 exists as a dimer, Fe2Cl6
(e) FeCl3 is used as stypic to stop bleeding from a cut.
(4) Ferrous sulphate, FeSO4, 7H2O (Green vitriol): It is prepared as follow,
Fe + H2SO4 → FeSO4 + H2
(i) One pressure to moist air crystals become brownish due to oxidation by air.
4FeSO4 + 2H2O + O2 → 4Fe(OH)SO4
(ii) On heating, crystals become anhydrous and on strong heating it decomposes to Fe2O3, SO2 andSO3.
heat strong
FeSO4.7H2O ———→ FeSO4 + 7H2O 2FeSO4 ————→ Fe2O3 + SO2 + SO3
heating
(iii) It can reduce acidic solution of KMnO4 and K2Cr2O7
(iv) It is generally used in double salt with ammonium sulphate.
(NH4)2SO4 + FeSO4 + 6H2O → FeSO4.(NH4)2SO4.6H2O
Mohr's salt
Mohr’s salt is resistant to atmospheric oxidation.
(v) It is used in the ring test for nitrate ions where it gives brown coloured ring of compound FeSO4.NO.
FeSO4 + NO → FeSO4 . NO
(vi) FeSO4 is used in manufacture of blue black ink.
(vii) FeSO4 + H2O2 is known as a name of Fenton’s reagent.
(5) Mohr's salt FeSO4.(NH4)2SO4.6H2O: It is a double salt and is prepared by crystallising a solution containing equivalent amounts of FeSO4.7H2O and (NH4)2SO4. It may be noted that Mohr’s salt contains only Fe2+ ions without any trace of Fe3+ ions. In contrast FeSO4.7H2O always contains some Fe3+ ions due to aerial oxidation of Fe2+ ions. Mohr salt is, therefore, used as a primary standard in volumetric analysis since a standard solution of Fe2+ ions can be obtained directly by weighing a known amount of the Mohr salt.
It acts as a reducing agent and as such reduces acidified KMnO4 and K2Cr2O7 solutions.
MnO4– + 5Fe2+ + 8H+ → 5Fe3+ + Mn2+ + 4H2O
Cr2O72– + 6Fe2+ + 14H+ → 6Fe3+ + 2Cr3+ + 7H2O
(From mohrs salt)
Compounds Of Mercury
Compounds of Mercury
(1) Mercuric oxide, HgO : It is obtained as a red solid by heating mercury in air or oxygen
for a long time at 673 K
679K
2Hg + O2 → 2 HgO ( red )
or by heating mercuric nitrate alone or in the presence of Hg
Heat
2Hg ( NO3 )2 → 2HgO + 4NO2 + O2
red
When NaOH is added to a solution of HgCI2, yellow precipitate of HgO are obtained.
Hg2 CI2 + 2NaOH → HgO ↓+ H2O + 2NaCl
(yello)
Red and yellow forms of HgO differ only in their particle size. On heating to 673 K, yellow form changes to red form.
673K
HgO → HgO
Yellow red
It is used in oil paints or as a mild antiseptic in ointments.
(2) Mercuric chloride, HgCl2 : It is obtained by treating Hg with CI2 or by heating a mixture of NaCland HgSO4 in presence of small amount of MnO2 (which oxidizes any Hg(I) salts formed during the reaction).
Heat
HgSO4 + 2NaCI → HgCI2 + Na2SO4
MnO2
It is a white crystalline solid and is commonly known as corrosive sublimate. It is a covalent compound since it dissolves in organic solvents like ethanol and ether.
It is extremely poisonous and causes death. Its best antidote is white of an egg.
When treated with stannous chloride, it is first reduced to white ppt. of mercurous chloride and then to mercury (black).
2HgCI2 + SnCI2 → Hg2CI2+ SnCI4
white ppt.
Hg2CI2 + SnCI2 → 2Hg + SnCI4
grey
With ammonia it gives a white ppt. known as infusible white ppt.
HgCI2 + 2NH3 → Hg ( NH2 ) CI + NH4 CI

 3HgSo4 → Hg2 SO4 + Hg + 2SO2 + 2O2
675K
3HgSo4 → Hg2 SO4 + Hg + 2SO2 + 2O2
675K
High temp
 Promotion of d-electrons to a higher level by
Promotion of d-electrons to a higher level by
A dilute solution of HgCI2 is used as an antiseptic.
(3) Mercuric iodide, HgI2 : It is obtained when a required amount of KI solution is added to a solution of . HgCI2
HgCI2 + 2KI → Hgl2 + 2KCl
(red)
Below 400 K, Hgl2 is red but above 400 K, it turns yellow
Hgl2 readily dissolves in excess of KI solution to form the ( Hgl4 )2- complex ion.
Hgl2 + 2Kl → K2Hgl4
Red ppt. soluble colourless solution
An alkaline solution of K2 [ Hgl4] is called Nessler’s reagent and is used to test NH+4 ions.
It gives a brown ppt. of NH2 - Hg - O Hg - I (Iodide of Millon’s base) with NH+4 ions.
2K2 [ HgI4 ] + NH3 + 3KOH → NH2. HgO. Hgl + 7KI + 2H2 O
It is used in ointments for treating skin infections.
(4) Mercurous chloride, Hg2Cl2 : It is obtained as under :
(a) Hg2 ( NO3 ) + 2NaCl → Hg2 Cl2 + 2NaNo3
white ppt.
Heat in an iron retort
(b) HgCl2 + Hg → Hg2 Cl2(condenses on cooling)
It is purified by sublimation.
Mercurous chloride is also called calomel. It is a white powder insoluble in H2O. On heating, it decomposes to give HgCl2 and Hg.
Heat
Hg2 Cl2 → Hgcl2 + Hg
It dissolves in chlorine water forming mercuric chloride.
Hg2Cl2 + Cl2 → 2HgCl2
With ammonia, it turns black due to the formation of a mixture of finely divided black Hg and mercuric amino chloride.
Hg2Cl2 + 2NH3 → Hg + NH2 HgCl + NH4Cl
(black)
It is used to prepare standard calomel electrode and as a purgative in medicine.
(5) Mercuric sulphide, HgS : The solubility product of HgS is lower than that of ZnS and hence it gets precipitated as black solid when H2S is passed through an acidic solution of any mercury (II) salt.
HgCl2 + H2S → HgS + 2HCl
It is insoluble in water and HCl but dissolves in aqua regia (1 part conc.HNO3 + 3 parts conc. HCl)
3HCl + HNO3 → NOCl + 2H2O + 2 [ Cl ]
Aqua regia Nitrosyl chloride Nacent chlorine
HgS + 2| Cl | → HgCl2 + S ↓
(Soluble)
On sublimation, its colour changes to red and hence it is used as a red pigment.
(6) Mercuric sulphate, HgSO4 : It is obtained when HgS is treated with conc.H2SO4.
Hg + 2H2 SO4 → HgSo4 + SO2 + 2H2O
It is a white solid which decomposes on heating to give mercurous sulphate.
It is used as a catalyst in the hydration of alkynes to give aldehydes or ketones. It is also used as a cosmetic under the name Vermillon and in ayurvedic medicine as makardhwaj.
(7) Amalgams : Mercury forms alloys commonly known as amalgams, with all metals except iron and platinum. Hence it is transported in iron containers.
.
Compounds of Silver
Compounds of Silver:
(1) Silver oxide (Ag2O): It is unstable and decomposes into Ag and O2 on slow heating.
2Ag2O → 4Ag + O2
(2) Silver halides (AgF, AgCl, AgBr and Agl) : Only AgF is soluble in H2O. AgCl is insoluble in H2O but dissolves in NH4OH, Na2S2O3 and KCN solutions. AgBr is partly soluble whereas Agl is completely insoluble in NH4OH. Except AgF, all the remaining three silver halides are photosensitive.
AgCl + 2NH4OH → [Ag(NH3)2]Cl + 2H2O
Diamine silver (I) chloride
AgCl + 2KCN → K [Ag(CN)2] + KCl
Pot.Dicyano argentate (I)
AgCl + 2Na2S2O3 → Na3[Ag(S2O3)2] + NaCl
Sod. Dithiosulphato argentate (I)
(3) Silver nitrate (AgNO3) : Silver nitrate (AgNO3) is called lunar caustic silver nitrate on heating above its m.p. (485 K) decomposes to silver nitrite but on heating to red heat gives silver.
Above 485 K
2AgNO3 ——————→ 2AgNO2 + O2
2AgNO3 → 2AG + 2NO2 + O2
When treated with alkali, AgNO3 forms silver oxide which in case of NH4OH dissolves to form complex ion.
2AgNO3 + 2NaOH → Ag2O + 2NaNO3 + H2O
2AgNO3 + 2NH4OH → Ag2O + 2NH4NO3 + H2O
Ag2O + 4NH4OH → 2[Ag(NH3)2]OH + 3H2O Diamine silver hydroxide
AgNO3 reacts with iodine in two ways
6AgNO3 (excess) + 3l2 + 3H2O → AglO3 + 5Agl + 6HNO3
5AgNO3 + 3l2 (excess) + 3H2O → HIO3 + 5Agl + 5HNO3
In contact with organic matter (skin, cloth, paper etc.) AgNO3 is reduced to metallic silver (black)
2AgNO3 + H2O → 2Ag + 2HNO3 + [O] → oxidises organic matter
AgNO3 gives different coloured ppt. with different anions like Cl–, Br–, I–, S2–, S2O32–, CrO43–, PO43–etc.
AgNO3 is used in the preparation of ink and hair dyes.
Photography : The photographic plate is coated with a colloidal gelatinised solution of AgBr. During exposure, AgBr is reduced to metallic silver.
2AgBr → 2Ag + Br2
The exposed film is developed. The developer used is an alkaline solution of hydroquinone or quinol which reduces some of the exposed AgBr to black silver.
C6H4(OH)2 + 2AgBr → 2Ag + C6H4O2 + 2HBr
Quinol Quinone
The film is finally fixed by dipping in a solution of sodium thiosulphate or hypo which removes unchanged AgBr as complex ion.
AgBr + 2Na2S2O3 → Na3[Ag(S2O3)2] + NaBr
After taking a print of the photograph it is finally toned by dipping in a dilute solution of gold chloride to impart a beautiful golden colour or it is dipped in potassium chloro platinate K2PtCl6 solution to get a shining grey tinge.
AuCl3 + 3Ag → 3AgCl + Au
Copper Ores
Copper Ores and its Extraction
(1) Ores: Copper pyrites (chalcopyrite) CeFeS2 Cuprite (ruby copper) Cu2O Copper glance (Cu2S), Malachite [Cu(OH)2.CuCO3] Azurite [Cu(OH)2.2CuCO3]
(2) Extraction : Most of the copper (about 75%) is extracted from its sulphide ore, copper pyrites.
Concentration of ore : Froth floatation process.
Roasting: Main reaction:
2CuFeS2 + O2 → Cu2S + 2FeS + SO2.
Side reaction:
2Cu2S + 3O2 → 2Cu2O + 2SO2
2FeS + 3O2 → 2FeO + 2SO2.
Smelting:
FeO + SiO2 → FeSiO3 (slag)
Cu2O + FeS → FeO + Cu2S
The mixture of copper and iron sulphides melt together to form 'matte' (Cu2S + FeS) and the slag floats on its surface.
Conversion of matte into Blister copper (Bessemerisation): Silica is added to matte and a hot blast of air is passed FeO + SiO2 → FeSiO3 (slag). Slag is removed. By this time most of iron sulphide is removed.
Cu2S + 2Cu2O → 6Cu + SO2
Blister copper: Which contain about 98% pure copper and 2% impurities (Ag, Au, Ni, Zn etc.)
Properties of copper: It has reddish brown colour. It is highly malleable and ductile. It has high electrical conductivity and high thermal conductivity. Copper is second most useful metal (first being iron). It undergoes displacement reactions with lesser reactive metals e.g. with Ag. It can displace Ag from AgNO3. The finally divided Ag so obtained is black in colour.
Copper shows oxidation states of +1 and +2. Whereas copper (I) salts are colourless, copper (II) salts are blue in colour. Cu (I) salts are less stable and hence are easily oxidised to Cu (II) salts (2Cu+ → Cu2+ + Cu) . This reaction is called disproportionation.
(1) In presence of atmospheric CO2 and moisture, copper gets covered with a green layer of basic copper carbonate (green layer) which protects the rest of the metal from further acton.
Cu + O2 + CO2 + H2O → Cu(OH)2 CuCO3
green layer
(2) In presence of oxygen or air, copper when heated to redness (below 1370K) first forms red cuprous oxide which changes to black cupric oxide on further heating. If the temperature is too high, cupric oxide changes back to cuprous oxide
Below 1370K O2
4Cu + O2 ————————→ 2Cu2O ————————→ 4CuO
(Red) Above 1370K (Black)
CuO + Cu ————————→ Cu2O
(3) Action of acids. Non oxidising dil. acids such as HCl, H2SO4 have no action on copper. However, copper dissolves in these acids in presence of air.
Cu + 2HCl + 1/2 O2(air) → CuCl2 + H2O
With dil. HNO3, Cu liberates NO (nitric oxide)
3Cu + 8HNO3 →, copper gives NO2
Cu + 4HNO3 → 3Cu(NO3)2 + 2NO2 + 2H2O
With hot conc. H2SO4, copper gives SO2
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Electrode potential of D block Elements
Electrode potentials(Eo)
Standard electrode potentials of some half–cells involving 3d-series of transition elements and their ions in aqueous solution are given in table,
Standard electrode potentials for 3d-elements
Elements
|
Ion
|
Electrode reaction
|
E°/ volt
|
Sc
|
Sc3+
|
Sc3++ 3e– Sc
|
– 2.10
|
Ti
|
Ti2+
|
Ti2++ 2e– Ti
|
– 1.60
|
V
|
V2+
|
V2++ 2e– V
|
– 1.20
|
Cr
|
Cr3+
|
Cr3+ + 3e– Cr
|
– 0.71
|
Mn
|
Mn2+
|
Mn2++ 2e– Mn
|
– 1.18
|
Fe
|
Fe2+
|
Fe2+ + 2e– Fe
|
– 0.44
|
Co
|
Co2+
|
Co2+ + 2e– Co
|
– 0.28
|
Ni
|
Ni2+
|
Ni2+ + 2e– Ni
|
– 0.24
|
Cu
|
Cu2+
|
Cu2+ + 2e– Cu
|
+ 0.34
|
Zn
|
Zn2+
|
Zn2+ + 2e– Zn
|
– 0.76
|
The negative values of E° for the first series of transition elements (except for Cu2+/ Cu ) indicate that,
(i) These metals should liberate hydrogen from dilute acids i.e., the reactions,
M + 2H+ → M2+ + H2 (g); 2M + 6H+ → 2M3+ + 3H2(g)
are favourable in the forward direction. In actual practice however, most of these metals react with dilute acids very slowly. Some of these metals get coated with a thin protective layer of oxide. Such an oxide layer prevents the metal to react further.
(ii) These metals should act as good reducing agents. There is no regular trend in the E° values. This is due to irregular variation in the ionisation and sublimation energies across the series.
Relative stabilities of transition metal ions in different oxidation states in aqueous medium can be predicted from the electrode potential data. To illustrate this, let us consider the following,
M(s) → M(g) ; ?H1 = Enthalpy of sublimation, ?Hsub
M(g) → M+(g) + e– ; ?H2 = Ionisation energy, IE
M+(g) → M+(aq) ; ?H3 = Enthalpy of hydration, ?Hhyd
Adding these equations one gets,
M(s) → M+(aq) + e–
?H = ?H1 + ?H2 + ?H3 = ?Hsub + IE + ?Hhyd
The represents the enthalpy change required to bring the solid metal M to the monovalent ion in aqueous medium, M+(aq).
The reaction, M(s) → M+(aq) + e–, will be favourable only if ?H is negative. More negative is the value is of ?H, more favourable will be the formation of that cation from the metal. Thus, the oxidation state for which ?H value is more negative will be stable in the solution.
Electrode potential for a Mn+/M half-cell is a measure of the tendency for the reaction, Mn+(aq) + ne–→ M(s)
Thus, this reduction reaction will take place if the electrode potential for Mn+/M half- cell is positive. The reverse reaction, M(s) → Mn+(aq) + ne–
Involving the formation of Mn+(aq) will occur if the electrode potential is negative, i.e., the tendency for the formation of Mn+(aq) from the metal M will be more if the corresponding E° value is more negative. In other words, the oxidation state for which E° value is more negative (or less positive) will be more stable in the solution.
When an elements exists in more than one oxidation states, the standard electrode potential (E°) values can be used in the predicting the relative stabilities of different oxidation states in aqueous solutions. The following rule is found useful.
The oxidation state of a cation for which ?H = (?Hsub + lE + ?Hhyd) or E° is more negative (for less positive) will be more stable.
1. Which of the following will have standard oxidation potential less than
(a) Zn (b) Cu
(c) Fe (d) Ni
Ans: b\
Enthalpy of Atomization
Enthalpy of atomization
Transition metals exhibit high enthalpies of atomization.
Explanation : This is because the atoms in these elements are closely packed and held together by strong metallic bonds. The metallic bond is formed as a result of the interaction of electrons in the outermost shell. Greater the number of valence electrons, stronger is the metallic bond.
Solved example 1: Which one of the following characteristics of the transition metals is associated with their catalytic activity
(a) Variable oxidation states
(b) High enthalpy of atomization
(c) Paramagnetic behaviour
(d) Colour of hydrated ions
Ans: a
Formation of colored ions
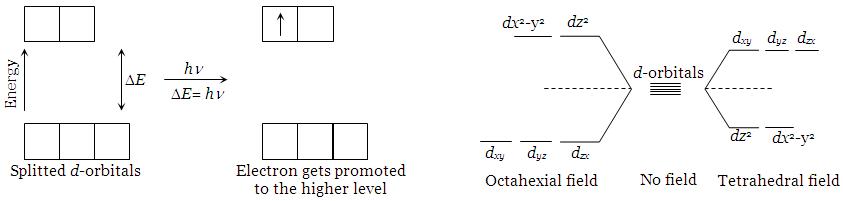
Most of the compound of the transition elements are colored in the solid state and /or in the solution phase. The compounds of transition metals are colored due to the presence of unpaired electrons in their d-orbitals.
Explanation : In an isolated atom or ion of a transition elements, all the five d-orbitals are of the same energy (they are said to be regenerate). Under the influence of the combining anion (s), or electron- rich molecules, the five d-orbitals split into two (or sometimes more than two) levels of different energies. The difference between the two energy levels depends upon the nature of the combining ions, but corresponds to the energy associated with the radiations in the visible region, (λ = 380 – 760nm). Typical splitting for octahedral and tetrahedral geometries are shown in fig.

The splitting of d-orbital energy levels in (a) an octahedral, (b) a tetrahedral, geometry. This spllitting is termed as the crystal field splitting.
The transition metals in elements form or in the ionic form have one or more unpaired electrons. When visible light falls on the sample, the electrons from the lower energy level get promoted to a higher energy level due to the absorption of light of a characteristic wavelength (or color). This wavelength (or color) of the absorbed light depends upon the energy difference of the two levels. Rest of the light gets transmitted. The transmitted light has a color complementary to the absorbed color. Therefore, the compound or the solution appears to be of the complementary color. For example, Cu(H2O)62+ ions absorb red radiation, and appear blue-green (blue-green is complementary color to red). Hydrated Co2+ ions absorb radiation in the blue-green region, and therefore, appear red in sunlight. Relationship between the color of the absorbed radiation and that of the transmitted light is given in table.
Relationship between the colors of the absorbed and transmitted light: the complementary colors.
Color of the
|
Color of the
| ||
Absorbed light
|
Transmitted light
|
Absorbed light
|
Transmitted light
|
IR
|
White
|
Blue-green
|
Red
|
Red
|
Blue-green
|
Blue
|
Orange
|
Orange
|
Blue
|
Indigo
|
Yellow
|
Yellow
|
Indigo
|
Violet
|
Yellow-green
|
Yellow-green
|
Violet
|
UV
|
White
|
Green
|
Purple
| ||
However, if radiations of all the wavelengths (or colors) except one are absorbed, then the color of the substance will be the color of the transmitted radiation. For example, if a substance absorbs all colors except green, then it would appear green to the eyes.
The transition metal ions which have completely filled d-orbitals are colorless, as there are no vacant d-orbitals to permit promotion of the electrons. Therefore, Zn2+ (3d10), Cd2 + (4d10) and Hg2+(5d10) Sc 3+, Ti4+, Cu+ ions and Zn, Cd, Hg are colorless and diamagnetic. The transition metal ions which have completely empty d-orbitals are also colorless, Thus, Sc3+ and Ti4+.ions are colorless, unless a colored anion is present in the compound.
Colors and the outer- electronic configurations of the some important ions of the first transition series elements are given bellow,
Ion
|
Outer configuration
|
Number of unpaired electrons
|
Colour of the ion
|
Sc3+
|
3d0
|
0
|
Colourless
|
Ti3+
|
3d1
|
1
|
Purple
|
Ti4+
|
3d0
|
0
|
Colourless
|
V3+
|
3d2
|
2
|
Green
|
Cr3+
|
3d3
|
3
|
Violet
|
Mn2+
|
3d5
|
5
|
Light pink
|
Mn3+
|
3d4
|
4
|
Violet
|
Fe2+
|
3d6
|
4
|
Green
|
Fe3+
|
3d5
|
5
|
Yellow
|
Co3+
|
3d7
|
3
|
Pink
|
Ni2+
|
3d8
|
2
|
Green
|
Cu2+
|
3d9
|
1
|
Blue
|
Cu+
|
3d10
|
0
|
Colourless
|
Zn2+
|
3d10
|
0
|
Colourless
|
\
Formation of complex Ions
Formation of complex ions
Transition metals and their ions show strong tendency for complex formation. The cations of transition elements (d-block elements) form complex ions with certain molecules containing one or more lone-pairs of electrons, viz., CO, NO, NH3 etc., or with anions such as, F–, Cl–, CN– etc. A few typical complex ions are,
[Fe(CN)]4–, [Cu(NH3)4]2+, [Y(H2O)6]2+,
[Ni(CO)4], [Co(NH3)6]3+ [FeF6]3–
Explanation : This complex formation tendency is due to,
(i) Small size and high nuclear charge of the transition metal cations.
(ii) The availability to vacant inner d-orbitals of suitable energy.
Formation of interstitial compounds: Transition elements form a few interstitial compounds with elements having small atomic radii, such as hydrogen, boron, carbon and nitrogen. The small atoms of these elements get entrapped in between the void spaces (called interstices) of the metal lattice. Some characteristics of the interstitial compound are,
(i) These are non-stoichiometric compounds and cannot be given definite formulae.
(ii) These compounds show essentially the same chemical properties as the parent metals, but differ in physical properties such as density and hardness. Steel and cast iron are hard due to the formation of interstitial compound with carbon. Some non-stoichimetric compounds are, VSe0.98 (Vanadium selenide), Fe0.94O and titanium nitride.
Explanation : Interstital compounds are hared and dense. This is because, the smaller atoms of lighter elements occupy the interstices in the lattice, leading to a more closely packed structure. Due to greater electronic interactions, the strength of the metallic bonds also increases.
Gold and its compounds
Gold and its Compounds
(1) Occurrence of gold : Gold is mainly found in native state either as vein gold, placer gold or alluvial gold. It is also present to a small extent in the combined state as sulphide, telluride and arsenosulphide. It is considered to be the king of metal.
Some important ores of gold are:
(i) Calaverite, AuTe2 (ii) Sylvanite, AuAgTe2 and
(iii) Bismuth aurite, BiAu2
(2) Extraction of gold : (i) Mac-Arthur-Forest Cyanide process : The powdered gold ore, after concentration by Froth-floatation process, is roasted to remove easily oxidisable impurities of tellurium, arsenic and sulphur. The roasted ore is then treated with a dilute solution of KCN in presence of atmospheric oxygen when gold dissolves due to the formation of an aurocyanide complex.
4Au + 8KCN + 2H2O + O2 → 4K[Au(CN)2] + 4KOH
solution
The metal is then extracted by adding zinc dust.
2K[Au(CN)2] + Zn → K2[Zn(CN)4] + 2Au↓
ppt
(ii) Plattner’s chlorine process : The roasted ore is moistened with water and placed in wooden vats with false perforated bottoms. It is saturated with current of chlorine, gold chloride thus formed is leached with water and the solution is treated with a reducing agent such as FeSO4 or H2S to precipitate gold.
AuCl3 + 3FeSO4 → Au↓ + FeCl3 + Fe2(SO4)3
2AuCl3 + 3H2S → 6HCl + 3S + 2Au↓
The impure gold thus obtained contains impurities of Ag and Cu. The removal of Ag and Cu from gold is called parting. This is done by heating impure gold with conc. H2SO4 (or HNO3 when Ag and Cu dissolve leaving behind Au.
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
2Ag + 2H2SO4 → Ag2SO4 + SO2 + 2H2O
Properties of Gold: Gold is a yellow, soft and heavy metal. Gold and Ag are called noble metals since they are not attacked by atmospheric oxygen. However, Ag gets tarnished when exposed to air containing traces of H2S. Gold is malleable, ductile and a good conductor of heat and electricity.
Pure gold is soft. It is alloyed with Ag or Cu for making jewellery. Purity of gold is expressed in terms of carats. Pure gold is 24 carats. Gold ’14 carats’ means that it is an alloy of gold which contains 14 parts by weight of pure gold and 10 parts of copper per 24 parts by weight of the alloy. Thus the percentage of gold in ’14 carats” of gold is = 100/24 × 14 = 58.3%.
Most of the jewellery is made from 22 carat gold (91.66% pure gold). Gold is quite inert. It does not react with oxygen, water and acids but dissolves in aqua regia
3HCl + HNO3 → NOCl + 2H2O + 2Cl] × 3
Au + 3Cl → AuCl3] × 2
2Au + 9HCl + 3HNO3 → 2AuCl3 + 6H2O + 3NOCl
Auric chloride Nitrosyl chloride
Oxidation states of gold: The principal oxidation states of gold are + 1 and + 3 though + 1 state is more stable than + 3.
Compounds of gold
(1) Auric chloride, AuCl3 : It is prepared by passing dry Cl2 over finely divided gold powder at 573 K
573K
2Au + 3Cl2 ————→ 2AuCl3
It is a red coloured crystalline solid soluble in water and decomposes on heating to give gold (I) chloride and Cl2
Heat
AuCl3 ————→ AuCl + Cl2
It dissolves in conc. HCl forming chloroauric acid
AuCl3 + HCl → H[AuCl4]
Chloroauric acid is used in photography for toning silver prints and as an antidote for snake poisoning.
(2) Aurous sulphide, Au2S : It is prepared when H2S is passed through an acidified solution of potassium aurocyanide, K[Au(CN)2]
2K[Au(CN)2] + H2S → Au2S + 2KCN + 2HCN
It is a dark brown solid, not attached by dilute mineral acids and hence is probably the most stable gold compound.
Hydroboration of Alkenes
HYDROBORATION OF ALKENES: PREPARATION OF ALKANES
(a) On treatment with acetic acid
B2H6 CH3COOH
R – CH = CH2 —————→ (R – CH2 – CH2)3 B ——————→ R – CH2 – CH3
Alkene Trialkyl borane Alkane
(b) Coupling of alkyl boranes by means of silver nitrate
2B2H6 AgNO325oC
6[R – CH = CH2] —————→ [2R – CH2 – CH2 – ]3 B ——————→ 3[RCH2CH2 – CH2CH2R]
Example 1: A gas decolourised KMnO4 solution but gives no precipitate with ammoniacal cuprous chloride is or Which of the following gases does not give a precipitate with ammoniacal solution of silver nitrate but decolourizes KMnO4 (neutral or slightly alkaline)
(a) Ethane (b) Methane
(c) Ethene (d) Acetylene
Ans (c)
Example 2: A hydrocarbon containing 2 carbon atoms gives Sabatier and Senderen's reaction but does not give precipitate with ammoniacal silver nitrate solution. The hydrocarbon in the question is
(a) Ethane (b) Acetylene
(c) Ethylene (d) None of these
Ans (c)
Ionic Radius of D Block Elements
Ionic Radius
For ions having identical charges, the ionic radii decrease slowly with the increase in the atomic number across a given series of the transition elements.
Elements (m):
|
Ionic radius,(M2+)/pm
|
Pm:(M3+)/pm:
| ||
Sc
|
–
|
81
|
Ti
|
90
|
76
|
V
|
88
|
74
|
Cr
|
84
|
69
|
Mn
|
80
|
66
|
Fe
|
76
|
64
|
Co
|
74
|
63
|
Ni
|
72
|
–
|
Cu
|
69
|
–
|
Zn
|
74
|
–
|
Explanation :The gradual decrease in the values of ionic radius across the series of transition elements is due to the increase in the effective nuclear charge.
Solved example 1: Which of the following trivalent ion has the largest atomic radii in the lanthanide series
(a) La (b) Ce
(c) Pm (d) Lu
Ans: a
Solved example 2:The atomic radii of the elements are almost same of which series
(a) Fe – Co – Ni (b) Na – K – Rb
(c) F – Cl – Br (d) Li – Be – B
Ans: a
Ionization energy of D block Elements
Ionization Energy
The ionization energies of the elements of first transition series are given below:
Elements
|
I1
|
I2
|
I3
|
Sc
|
632
|
1245
|
2450
|
Ti
|
659
|
1320
|
2721
|
V
|
650
|
1376
|
2873
|
Cr
|
652
|
1635
|
2994
|
Mn
|
716
|
1513
|
3258
|
Fe
|
762
|
1563
|
2963
|
Co
|
758
|
1647
|
3237
|
Ni
|
736
|
1756
|
3400
|
Cu
|
744
|
1961
|
3560
|
Zn
|
906
|
1736
|
3838
|
* in kJ mol–1
The following generalizations can be obtained from the ionisation energy values given above.
(i) The ionisation energies of these elements are high and in the most cases lie between those of s- and p-block elements. This indicates that the transition elements are less electropositive than s-block elements.
Explanation : Transition metals have smaller atomic radii and higher nuclear charge as compared to the alkali metals. Both these factors tend to increase the ionisation energy, as observed.
(ii) The ionisation energy in any transition series increases in the nuclear with atomic number; the increase however is not smooth and as sharp as seen in the case of s and p-block elements.
Explanation : The ionisation energy increases due to the increase in the nuclear charge with atomic number at the beginning of the series. Gradually, the shielding effect of the added electrons also increases. This shielding effect tends to decrease the attraction due to the nuclear charge. These two opposing factors lead to a rather gradual increase in the ionisation energies in any transition series.
(iii) The first ionisation energies of 5d-series of elements are much higher than those of the 3d and 4d series elements.
Explanation : In the 5d-series of transitions elements, after lanthanum (La), the added electrons go to the next inner 4f orbitals. The 4f electrons have poor shielding effect. As a result, the outermost electrons experience greater nuclear attraction. This leads to higher ionisation energies for the 5d- series of transition elements.
Solved example 1: Ionisation potential values of d-block elements as compared to ionization potential value of f-block elements are
(a) Higher (b) Equal
(c) lower (d) All of these
Ans: a
Iron and its properties
(1) Ores of iron : Haematite Fe2O3, Magnetite (Fe3O4) Limonite (Fe2O3.3H2O), Iron pyrites (FeS2) Copper pyrities (CuFeS2) etc.
(2) Extraction : Cast iron isextracted from its oxides by reduction with carbon and carbon monoxide in a blast furnace to give pig iron.
Roasting : Ferrous oxide convert into ferric oxide.
Fe2O3.3H2O → Fe2O3 + 3H2O; 2FeCO3 → 2FeO + 2CO2
4FeO + O2 → 2Fe2O3
Smelting : Reduction of roasted ore of ferric oxide carried out in a blast furnace.
(i) The reduction of ferric oxide is done by carbon and carbon monoxide (between 1473k to 1873k) 2C + O2 → 2CO
673k
(ii) Fe2O3 + 3CO ? 2Fe + 3CO2. It is a reversible and exothermic reaction. Hence according toLe-chatelier principle more iron will be produced in the furnace at lower temp. Fe2O3 + CO → 2FeO + CO2
1073K
(iii) FeO + C ————————→ Fe + Co
endothermic reaction
The gases leaving at the top of the furnace contain up to 28% CO and are burnt in cowper's stove to pre-heat the air for blast
Varieties of iron : The three commercial varieties of iron differ in their carbon contents. These are;
(1) Cast iron or Pig-iron : It is most impure form of iron and contains highest proportion of carbon (2.5–4%).
(2) Wrought iron or Malleable iron : It is the purest form of iron and contains minimum amount of carbon (0.12–0.25%).
(3) Steel : It is the most important form of iron and finds extensive applications. Its carbons content (Impurity) is mid-way between cast iron and wrought iron. It contains 0.2–1.5% carbon. Steels containing 0.2–0.5% of carbon are known as mild steels, while those containing 0.5–1.5% carbon are known as hard steels.
Steel is generally manufactured from cast iron by three processes, viz, (i) Bessemer Process which involves the use of a large pear-shaped furnace (vessel) called Bessemer converter, (ii) L.D. process and (iii) open hearth process, Spiegeleisen (an alloy of Fe, Mn and C) is added during manufacture of steel.
Heat treatment of steels : Heat treatment of steel may be defined as the process of carefully heating the steel to high temperature followed by cooling to the room temperature under controlled conditions. Heat treatment of steel is done for the following two purposes,
(i) To develop certain special properties like hardness, strength, ductility etc. without changing the chemical composition.
(ii) To remove some undesirable properties or gases like entrapped gases, internal stresses and strains. The various methods of heat treatment are,
(a) Annealing : It is a process of heating steel to redness followed by slow cooling.
(b) Quenching or hardening : It is a process of heating steel to redness followed by sudden cooling by plunging the red hot steel into water or oil.
(c) Tempering : It is a process of heating the hardened or quenched steel to a temperature much below redness (473–623K) followed by slow cooling.
(d) Case-hardening : It is a process of giving a thin coating of hardened steel to wrought iron or to a strong and flexible mild steel by heating it in contact with charcoal followed by quenching in oil.
(e) Nitriding : It is a process of heating steels at about 700oC in an atmosphere of ammonia. This process imparts a hard coating of iron nitride on the surface of steel.
Properties of steel : The properties of steel depend upon its carbon contents. With the increase in carbon content, the hardness of steel increases while its ductility decreases.
(i) Low carbon or soft steels contain carbon upto 0.25%.
(ii) Medium carbon steels or mild steels contain 0.25–0.5% carbon.
(iii) High carbon or hard steels contains 0.1 – 1.5 percent carbon.
(iv) Alloy steels or special steels are alloys of steel with Ni, Cr, Co, W, Mn, V etc., For example
(a) Stainless steel (Fe = 73%, Cr = 18%, Ni = 8% + C) is resistant to corrosion and is used for making ornamental pieces, cutlery etc.
(b) Invar (Fe = 64%, Ni = 36%) has small coefficient of expansion and is used for making metre scales, pendulum rods and watches.
(c) Manganese steel (Fe = 86%, Mn 13% + carbon) is very hard and resistant to wear and hence is used for making rock drills, safes etc.
(d) Tungsten steel (Fe = 94%, W = 5% + carbon) is quite hard and is used for making high speed cutting tools.
(e) Permalloy (Fe = 21%, Ni = 78% + carbon) is strongly magnetised by electric current but loses magnetism when current is cut off. It is used for making electromagnets, ocean cables etc.
Properties of iron
(1) Dry or moist air has no action on pure iron but impure iron when exposed to moist air is covered with a layer of rust Fe2O3 + Fe(OH)3. However, finely divided pure iron burns in air or oxygen forming Fe3O4 (magnetic oxide of iron).
3Fe + 2O2 → Fe3O4
(2) Iron decomposes steam at red heat
Red heat
3Fe + 4H2O ——————→ Fe3O4 + 4H2
Steam
(3) Action of acids: Iron reacts with dil. HCl and dil. H2SO4 liberating hydrogen. with hot conc.H2SO4, it gives SO2, with dil.HNO3, it gives NH4NO3 and moderately conc. HNO3 reacts with iron forming NO2.
Cold conc. HNO3 makes iron passive due to the deposit of a thin layer of iron oxide (Fe3O4) on the surface.
Hot conc. HNO3 reacts with iron liberating NO.
Fe + 4HNO3 (hot conc.) → Fe(NO3)3 + NO + 2H2O
(4) Iron does not react with alkalies.
(5) It displaces less electropositive metals (e.g., Cu, Ag etc.) from their salts
CuSO4 + Fe → FeSO4 + Cu
(6) Finely divided iron combines with CO forming penta carbonyl
Fe + 5CO → Fe(CO)5
(7) Iron does not form amalgam with Hg.
(8) Iron is the most abundant and most widely used transition metal.
Lanthanides
F Block Elements & Lanthanides
Lanthanides and actinides are collectively called f-block elements because last electron in them enters into f-orbitals of the antepenultimate (i.e., inner to penultimate) shell partly but incompletely filled in their elementary or ionic states. The name inner transition, elements is also given to them because they constitute transition series with in transition series (d-block elements) and the last electron enters into antepenultimate shell (n-2)f. In addition to incomplete d-subshell, their f-subshell is also incomplete. Thus, these elements have three incomplete outer shells i.e., (n–2), (n–1) and n shells and the general electronic configuration of f-block elements is (n–2) f1-14 ( n- 1 ) d0-10 ns2.
(1) Lanthanides : The elements with atomic numbers 58 to 71 i.e. cerium to lutetium (which come immediately after lanthanum Z = 57) are called lanthanides or lanthanones or rare earths. These elements involve the filling of 4 f-orbitals. Their general electronic configuration is [ Xe ] 4f1-14 4d0-10 6s2,. Promethium (Pm), atomic number 61 is the only synthetic (man made) radioactive lanthanide.
Properties of lanthanides
(i) These are highly dense metals and possess high melting points.
(ii) They form alloys easily with other metals especially iron. e.g. misch metal consists of a rare earth element (94–95%), iron (upto 5%) and traces of S, C, Ca and Al, pyrophoric alloys contain Ce (40–5%),La + neodymium (44%), Fe (4–5%), Al (0–5%) and the rest is Ca, Si and C. It is used in the preparation of ignition devices e.g., trace bullets and shells and flints for lighters and cigarette.
(iii) Oxidation state : Most stable oxidation state of lanthanides is +3. Oxidation states + 2 and + 4 also exist but they revert to +3 e.g. Sm2+, Eu2+, Yb2+ lose electron to become +3 and hence are good reducing agents, where as Ce4+, Pr4+, Tb4+ in aqueous solution gain electron to become + 3 and hence are good oxidizing agents. There is a large gap in energy of 4 f and 5 d subshells and thus the number of oxidation states is limited.
(iv) Colour : Most of the trivalent lanthanide ions are coloured both in the solid state and in aqueous solution. This is due to the partly filled f-orbitals which permit f–f transition. The elements with xfelectrons have a similar colour to those of (14 – x) electrons.
(v) Magnetic properties : All lanthanide ions with the exception of Lu3+, Yb3+ and Ce 4+ are paramagnetic because they contain unpaired electrons in the 4 f orbitals. These elements differ from the transition elements in that their magnetic moments do not obey the simple “spin only” formula μeff= √n(n+2) B.M. where n is equal to the number of unpaired electrons. In transition elements, the orbital contribution of the electron towards magnetic moment is usually quenched by interaction with electric fields of the environment but in case of lanthanides the 4f-orbitals lie too deep in the atom for such quenching to occur. Therefore, magnetic moments of lanthanides are calculated by taking into consideration spin as well as orbital contributions and a more complex formula
μeff= √4S ( S+1 ) + L (L+1 ) B.M.
Which involves the orbital quantum number L and spin quantum number S.
(vi) Complex formation : Although the lanthanide ions have a high charge (+3) yet the size of their ions is very large yielding small charge to size ratio i.e., low charge density. As a consequence, they have poor tendency to form complexes. They form complexes mainly with strong chelating agents such as EDTA, β -diketones, oxine etc. No complexes with π - bonding ligands are known.
(vii) Lanthanide contraction : The regular decrease in the size of lanthanide ions from to is known as lanthanide contraction. It is due to greater effect of the increased nuclear charge than that of the screening effect.
Consequences of lanthanide contraction
(a) It results in slight variation in their chemical properties which helps in their separation by ion exchange
(b) Each element beyond lanthanum has same atomic radius as that of the element lying above it in the group (e.g. Zr 145 pm, Hf 144 pm); Nb 134 pm, Ta 134 pm ; Mo 129 pm, W 130 pm).
(c) The covalent character of hydroxides of lanthanides increases as the size decreases from La3+ to Lu3+ . However basic strength decreases. La (OH)3Thus is most basic whereas Lu (OH)3 is least basic. Similarly, the basicity of oxides also decreases in the order from La3+ to Lu3+.
(d) Tendency to form stable complexes from La3+ to Lu3+ increases as the size decreases in that order.
(e) There is a slight increase in electronegativity of the trivalent ions from La to Lu.
(f) Since the radius of Yb3+ ion (86 pm) is comparable to the heavier lanthanides Tb, Dy, Ho and Er, therefore they occur together in natural minerals.
Magnetic properties of D block Elements
Magnetic properties
Most of the transition elements and their compounds show paramagnetism. The paramagnetism first increases in any transition element series, and then decreases. The maximum paramagnetism is seen around the middle of the series. The paramagnetism is described in Bohr Magneton (BM) units. The paramagnetic moments of some common ions of first transition series are given below in Table
Explanation :A substance which is attracted by magnetic filed is called paramagnetic substance. The substances which are repelled by magnetic filed are, called diamagnetic substances. Paramagnetism is due to the presence of unpaired electrons in atoms, ions or molecules.
The magnetic moment of any transition element or its compound/ion is given by (assuming no contribution from the orbital magnetic moment).
μs = √4S(S+1) BM = √n(n+2) BM
where, S is the total spin (n×s) n is the number of unpaired electrons and s is equal to ½ (representing the spin of an unpaired electron).
From the equation given above, the magnetic moment (μs) increases with an increase in the number of unpaired electrons.
Magnetic moments of some ions of the 3d-series elements
Ion
|
Outer configuration
|
No. of unpaired electrons
|
Magnetic moment (BM)
| |
Calculated
|
observed
| |||
Sc3+
|
3d0
|
0
|
0
|
0
|
Ti3+
|
3d1
|
1
|
1.73
|
1.75
|
Ti2+
|
3d2
|
2
|
2.84
|
2.86
|
V2+
|
3d3
|
3
|
3.87
|
3.86
|
Cr2+
|
3d4
|
4
|
4.90
|
4.80
|
Mn2+
|
3d5
|
5
|
5.92
|
5.95
|
Fe2+
|
3d6
|
4
|
4.90
|
5.0-5.5
|
Co2+
|
3d7
|
3
|
3.87
|
4.4-5.2
|
Ni2+
|
3d8
|
2
|
2.84
|
2.9-3.4
|
Cu2+
|
3d9
|
1
|
1.73
|
1.4-2.2
|
Zn2+
|
3d10
|
0
|
0
|
0
|
In d-obitals belonging to a particular energy level, there can be at the maximum five unpaired electrons in d5 cases. Therefore, paramagnetism in any transition series first increases, reaches a maximum value for d5 cases and then decreases thereafter.
Example 1: Which ion has maximum magnetic moment
(a) V+3 (b) Mn+3
(c) Fe+3 (d) Cu+2
Ans: c
Manganese Containing Compounds
Manganese containing compounds
Potassium Permanganate, (KMnO4)
Potassium permanganate is a salt of an unstable acid HMnO4 (permanganic acid). The Mn is an +7 state in this compound.
Preparation : Potassium permanganate is obtained from pyrolusite as follows.
Conversion of pyrolusite to potassium manganate : When manganese dioxide is fused with potassium hydroxide in the presence of air or an oxidising agent such as potassium nitrate or chlorate, potassium manganate is formed, possibly via potassium manganite.
fused
MnO2 + 2KOH ––––––→ K2MnO3 + 4H2O] × 2
potassium manganite
2K2MnO3 + O2 ––––––→ 2K2MnO4 + 2H2O
fused
2MnO2 + 4KOH + O2 ––––––→ 2K2MnO4 + 2H2O
pyrolusite potassium manganate
[dark-green mass]
Oxidation of potassium manganate to potassium permanganate: The potassium manganate so obtained is oxidized to potassium permanganate by either of the following methods.
By chemical method : The fused dark-green mass is extracted with a small quantity of water. The filtrate is warmed and treated with a current of ozone, chlorine or carbon dioxide. Potassium manganate gets oxidized to potassium permanganate and the hydrated manganese dioxide precipitates out. The reactions taking place are,
When CO2 is passed
3K2MnO4 + 2H2O → 2KMnO4 + MnO2 ↓ + 4KOH
potassium manganate potassium permanganate
2CO2 + 4KOH → 2K2CO3 + 2H2O
When chlorine or ozone is passed
2K2MnO4 + Cl2 → 2KMnO4 + 2KCl
2K2MnO4 + O3 + H2O → 2KMnO4 + 2KOH + O2(g)
The purple solution so obtained is concentrated and dark purple, needle-like crystals having metallic lustre are obtained.
Electrolytic method : Presently, potassium manganate (K2MnO4) is oxidized electrolytically. The electrode reactions are,
At anode: 2MnO42– → 2MnO4– + 2e–
At cathode: 2H+ + 2e– → H2(g)
The purple solution containing KMnO4 is evaporated under controlled condition to get crystalline sample of potassium permanganate.
Physical properties
KMnO4 crystallizes as dark purple crystals with greenish luster (m.p. 523 K).
It is soluble in water to an extent of 6.5g per 100g at room temperature. The aqueous solution of KMnO4has a purple colour.
Melting and Boiling Points
MELTING AND BOILING POINTS OF D BLOCK ELEMENTS
The melting and boiling points of transition elements except Cd and Hg, are very high as compared to the s-block and p-block elements. The melting and boiling points first increase, pass through maxima and then steadily decrease across any transition series. The maximum occurs around middle of the series.
Explanation : Atoms of the transition elements are closely packed and held together by strong metallic bonds which have appreciable covalent character. This leads to high melting and boiling points of the transition elements.
The strength of the metallic bonds depends upon the number of unpaired electrons in the outermost shell of the atom. Thus, greater is the number of unpaired electrons stronger is the metallic bonding. In any transition element series, the number of unpaired electrons first increases from 1 to 5 and then decreases back to the zero .The maximum five unpaired electrons occur at Cr (3d series). As a result, the melting and boiling points first increase and then decrease showing maxima around the middle of the series.
The low melting points of Zn, Cd, and Hg may be due to the absence of unpaired d-electrons in their atoms.
Solved example 1: Mercury is the only metal which is liquid at 0oC. This is due to its
(a) Very high ionisation energy and weak metallic bond
(b) Low ionisation potential
(c) High atomic weight
(d) High vapour pressure
Ans: a
Mercury Ores
Mercury Ores and Extraction
(1) Occurrence and extraction of mercury: Cinnabar (HgS) is the only important ore of Hg. It is concentrated by froth floatation method and mercury is extracted from this ore by heating it in air at 773-873 K (auto reduction).
273-873K
HgS + O2 → Hg + SO2
The mercury vapours thus obtained are condensed to give liquid metal. Hg thus obtained contains impurities of Zn, Sn and Pb. These are removed by treating the impure metal with dil HNO3 , mercurous nitrate, Hg2 ( NO3 )2 thus formed react with metals present as impurities forming their nitrates which pass into solution leaving behind pure mercury. However, it is best purified by distillation under reduced pressure.
warm
6Hg + 8HNO3 (dil. ) → 3 Hg ( NO3 )2 + 4H2O + 2NO
Zn + Hg2 ( NO3 )2 → Zn ( NO3 )2 + 2Hg
Similar reaction is given by Pb and Sn.
Properties of mercury : Mercury is less reactive than Zn. It is a liquid at room temperature and has low thermal and electrical conductivity. Mercury forms dimeric mercury (I) ions, Hg+22 in which the two atoms are bonded by a covalent bond. It is slowly oxidised to HgO at about its boiling point. Hg does not react with dil. HCl or dil. H2SO4 but reacts with hot concentrated H2SO4 to form HgSO4, it reacts with both warm dil. and conc. HNO3 evolving NO and NO2 respectively.
Hg + 2H2SO4 ( hot, conc. ) → HgSO4 + SO2 + 2H2O
Hg + 4HNO3 ( conc. ) → Hg ( NO3 )2 +2NO2 + 2H2O
Hg does not react with steam or water hence can’t form any hydroxide.
Metallic character of D block Elements
METALLIC CHARACTER OF D BLOCK ELEMENTS
All the transition elements are metals. These are hard, and good conductor of heat and electricity. All these metals are malleable, ductile and form alloys with other metals. These elements occur in three types e.g., face- centered cubic (fcc), hexagonal close-packed (hcp) and body-centered cubic (bcc), structures.
The transition elements shows both covalent as well as metallic bonding amongst their atoms.
Explanation : The ionisation energies of the transition elements are not very high. The outermost shell in their atoms have many vacant, partially filled orbitals. These characteristics make these elements metallic in character. The hardness of these metals, suggests the presence of covalent bonding in these metals. The presence of unfilled d-orbitals favour covalent bonding. Metallic bonding in these metals is indicated by the conducting nature of these metals. Therefore, it appears that there exists covalent and metallic bonding in transition elements.
Solved Example 1: In human body if necessary, the plate, screw or wire used for surgery are made up of
(a) Ni (b) Au
(c) Pt (d) Ta
Ans: d
Solved Example 2: A hard and resistant metal (alloy) generally used in tip of nib of fountain pen is
(a) Os.lr (b) Pt.Cr
(c) V.Fe (d) Fe.Cr
Ans : b
Silver Ores and Extraction
Silver ores and its extraction
(1) Ores : Argentite (silver glance) Ag2S Horn silver (AgCl) Ruby silver (Pyrargyrite) 3Ag2S.Sb2S3.
(2) Extraction : Cyanide process or Mac Arthus-Forrest cyanide process : This method depends on the fact that silver, its sulphide or chloride, forms soluble complex with alkali cyanides in the silver. This implies that silver compounds will dissolve in solution of alkali cyanides in the presence of blast of air.
4Ag + 8NaCN + 2H2O + O2 ? 4Na[Ag(CN)2] + 4NaOH
air
or 4Ag + 8CN– + 2H2O + O2 ? 4[Ag(CN)2]– + 4OH–
Ag2S + 4NaCN ? 2Na[Ag(CN)2] + Na2S
AgCl + 2NaCN ? Na[Ag(CN)2] + NaCl
The reaction with the sulphide is reversible and accumulation of Na2S must be prevented. A free excess of air is continuously passed through the solution which oxidizes Na2S into sulphate and thiosulphate.
2Na2 + 2O2 + H2O → Na2S2O3 + 2NaOH
Na2S2O3 + 2NaOH + 2O2 → 2Na2SO4 + H2O
2Na[Ag(CN)2] + 4NaOH + Zn → Na2ZnO2 + 4NaCN + 2H2O + 2Ag
(3) Extraction of Ag from argentiferrous lead (PbS + Ag2S)– Parke’s Process : It is based upon the following facts (i) Molten Zn and Pb are immiscible, zinc forms the upper layer (ii) Ag is more soluble in molten Zn (iii) Zn-Ag alloy solidifies earlier than molten Pb (IV) Zn being volatile can be separated from Ag by distillation. Ag is purified by cupellation.
Properties of Silver: Silver is a white lustrous metal, best conductor of heat and electricity. Being soft, it is alloyed. The silver alloy used for making jewellery contain 80% Ag and 20% Cu. The composition of a silver alloy is expressed as its purity i.e. the amount of Ag present in 1000 parts of the alloy Ag does not react with dilute HCl or dil. H2SO4 and aqua regia but reacts with dil. HNO3 and conc. HNO3 forming NO and NO2 respectively. Chlorine also reacts with Ag to form AgCl.
2Ag + Cl2 → 2AgCl
Hot conc. H2SO4 reacts with Ag forming SO2 like Cu
Zinc and its Compounds
Zinc and its Compounds
(1) Occurrence of zinc: Zinc does not occur in the native form since it is a reactive metal. The chief ores of zinc are (i) Zinc blende (ZnS) (ii) Calamine or zinc spar (ZnCO3) and (iii) Zincite (ZnO)
(2) Extraction of zinc : Zinc blende, after concentration by Froth floatation process, is roasted in air to convert it into ZnO. In case of calamine, ore is calcined to get ZnO. The oxide thus obtained is mixed with crushed coke and heated at 1673 K in fire clay retorts (Belgian Process) when ZnO gets reduced to metallic zinc. Being volatile at this temperature, the metal distils over and is condensed leaving behind Cd, Pb and Fe as impurities. The crude metal is called spelter. The metal may be refined either by electrolysis or by fractional distillation.
Properties of Zn : Zinc is more reactive than mercury. It is a good conductor of heat and electricity. Zinc readily combines with oxygen to form ZnO. Pure zinc does not react with non-oxidising acids (HCl or H2SO4) but the impure metal reacts forming Zn2+ ions and evolving H2 gas.
Zn + 2HCl → ZnCl2 + H2↑
Hot and conc. H2SO4 attacks zinc liberating SO2 gas
Zn + 2H2SO4 → ZnSO4 + SO2 + 2H2O
Zinc also reacts with both dilute (hot and cold) HNO3 and conc. HNO3 liberating nitrous oxide (N2O), ammonium nitrate (NH4NO3) and nitrogen dioxide (NO2) respectively.
4Zn + 10HNO3 (warm, dilute) → 4Zn(NO3)2 + N2O + 5H2O
4Zn + 10HNO3 (cold very dilute) → 4Zn(NO3)2 + 2NO2 + 2H2O
Zn + 4HNO3 (hot and conc.) → Zn(NO3)2 + 2N2O + 2H2O
Zinc dissolves in hot concentrated NaOH forming the soluble sod. Zincate
Zn + 2NaOH + 2H2O → Na2 [Zn(OH)4] + H2
Or Zn + 2NaOH → Na2ZnO2 + H2
(3) Special varieties of zinc. (i) Zinc dust : It is prepared by melting zinc and then atomising it with a blast of air.
(ii) Granulated zinc : It is prepared by pouring molten zinc into cold water.
Both these varieties of zinc are used as reducing agents in laboratory.
Compounds of zinc
(1) Zinc oxide (Zinc white or Chinese white), ZnO : It is obtained by burning zinc in air or by heating zinc carbonate or zinc nitrate.
2Zn + O2 → 2ZnO
ZnCO3 → ZnO + CO2
2Zn(NO3)2 → 2ZnO + 4NO2 + O2
It is a white powder but becomes yellow and heating and again white on cooling.
It is insoluble in water and is vey light and hence commonly known as philosopher's wool.
It is amphoteric in nature.
ZnO + 2HCl → ZnCl + H2O
(Black)
ZnO + 2NaOH → Na2ZnO2 + H2O
(Acidic) Sod. sincate
Or ZnO + 2NaOH + H2O → Na2[Zn(OH)4]
Sod. tetrahydrozincate(II)
It is reduced both by carbon adn H2 and is used as a white paint
ZnO + C → Zn + CO; ZnO + H2 → Zn + H2O
(2) Zinc chloride, ZnCl2 : It is obtained when Zn metal, ZnO or ZnCO3 is treated with dil. HCl. It crystallizes as ZnCl2.2H2O and becomes anhydrous on heating. ZnCl2 is highly deliquescent and is highly soluble in H2O and also readily dissolves in organic solvents like acetone, alcohol, ether etc. its aqueous solution is acidic due to hydrolysis.
ZnCl2 + H2O → Zn(OH)Cl + HCl
Anhydrous ZnCl2 is used as a Lewis acid catalyst in organic reactions. Mixed with moist zinc oxide, it is used for filling teeth and its solution is used for preserving timber. Anhydrous ZnCl2 used as a Lucas reagent with conc. HCl.
(3) Zinc sulphide, ZnS : It is a white solid. It is soluble in dil. HCl and thus does not get precipitated by H2S in the acidic medium.
ZnS + 2HCl → ZnCl2 + H2S
It is a constituent of lithopone (ZnS + BaSO4)
(4) Zinc sulphate, ZnSO4.7H2O : It is commonly known as white vitriol and is obtained by the action of dil. H2SO4 on zinc metal, ZnO or ZnCO3. On heating, it first loses six molecules of water of crystallization at 373 K. At 723 K, it becomes anhydrous and on further heating, it decomposes.
373K 723K
ZnSO4.7H2O —————→ ZnSO4.H2O ————→
1073K
2ZNSO4 —————→ 2ZnO + 2SO2 + O2
It is used to prepare lithopone (BaSO4 + ZnS), a white paint and also in galvanishing iron.
ZnSO4 + BaS → ZnS + BaSO4

No comments:
Post a Comment